Epidemiology
Type 2 DM
- Age
- Adult onset typically > 40 years
- Mean age of onset is decreasing
- Race: highest prevalence in Native Americans, Hispanics, African Americans, and Asian non-Hispanic Americans
Etiology
Type 1 DM
- Autoimmune destruction of pancreatic β cells in genetically susceptible individuals
- HLA association: HLA-DR3 and HLA-DR4 positive patients are at increased risk of developing T1DM.
- Associated with other autoimmune conditions
- Hashimoto thyroiditis
- Type A gastritis
- Celiac disease
- Primary adrenal insufficiency
Classifications
- Type 1: formerly known as insulin-dependent (IDDM) or juvenile-onset diabetes mellitus
- Type 2: formerly known as non-insulin-dependent (NIDDM) or adult-onset diabetes mellitus
- Gestational diabetes
- Other types of diabetes mellitus
- MODY (maturity-onset diabetes of the young): genetic defects leading to β-cell dysfunction
- Different forms of autosomal dominant inherited diabetes mellitus that manifest before the age of 25 years and are not associated with obesity or autoantibodies
- Multiple monogenic subtypes (most common: MODY II due to glucokinase gene defect, and MODY III, due to hepatocyte nuclear factor-1-α gene defect)
- MODY II
- A single mutation leads to impaired insulin secretion due to altered glucokinase function.
- Glucokinase is the glucose sensor of the β cell, facilitating storage of glucose in the liver, especially at high concentrations.
- There is no increased risk of microvascular disease.
- Despite stable hyperglycemia and chronically elevated HbA1C levels, MODY II can be managed with diet alone.
Pathophysiology
Normal insulin physiology
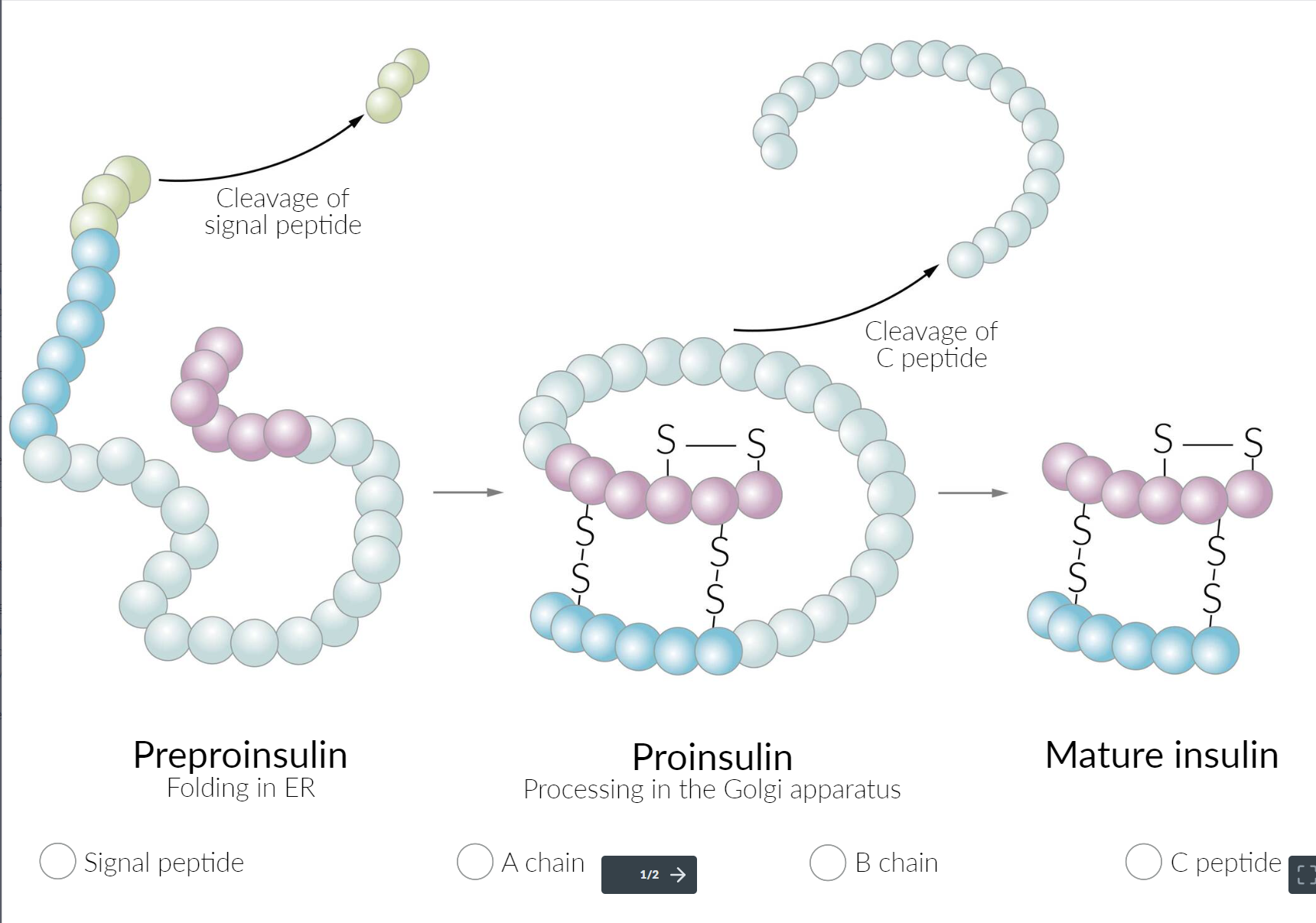
- Secretion: Insulin is synthesized in the β cells of the islets of Langerhans. The cleavage of proinsulin (precursor molecule of insulin) produces C-peptide (connecting peptide) and insulin, which consists of two peptide chains (A and B chains).
- Action
- Carbohydrate metabolism
- Protein metabolism: insulin inhibits proteolysis, stimulates protein synthesis, and stimulates cellular uptake of amino acids
- Lipid metabolism: maintains a fat depot and has an antiketogenic effect
- Electrolyte regulation:
- Stimulates intracellular potassium accumulation
- Directly stimulates Na+/K+ ATPase and promotes intracellular alkalosis, reduces phosphate levels (glucose binds to phosphate in the cell), and stimulates magnesium uptake into cells
- Insulin boosts glucose uptake into cells through two linked processes:
- Direct effect: Insulin stimulates GLUT transporters to move glucose into cells through facilitated diffusion
- Indirect effect: Insulin activates Na⁺/K⁺-ATPase pumps, which:
- Pump Na⁺ out of cells → Creates high Na⁺ outside
- This Na⁺ gradient powers SGLT transporters
- SGLT uses Na⁺ flow into cells to drag glucose in with it (co-transport)
- Reduces phosphate levels: When insulin promotes glucose uptake, glucose must be phosphorylated to glucose-6-phosphate
- Stimulates intracellular potassium accumulation
- Those with long-standing diabetes (ie, >5 years) frequently also have alpha cell failure with decreased glucagon secretion and therefore have an even greater risk of rapid hypoglycemia.
Type 1 diabetes
- Core Defect: Autoimmune destruction of pancreatic β-cells (Type IV hypersensitivity).
- Mediated primarily by CD8+ cytotoxic T-lymphocytes. t
- Strong genetic link: HLA-DR3 and HLA-DR4.
- Resulting State: Absolute insulin deficiency.
- Unopposed glucagon action leads to:
- ↑ Hepatic glucose production (gluconeogenesis, glycogenolysis) → Hyperglycemia.
- ↑ Lipolysis & proteolysis → Ketone body production (DKA) & weight loss.
- Unopposed glucagon action leads to:
- Key Markers & Pathology:
- Autoantibodies: Anti-GAD-65, anti-islet cell.
- Histology: Insulitis (lymphocytic infiltrate in islets).
Type 2 diabetes
- Mechanisms
- Peripheral insulin resistance
- Numerous genetic and environmental factors
- Central obesity → increased plasma levels of free fatty acids → impaired insulin-dependent glucose uptake into hepatocytes, myocytes, and adipocytes
- Increased serine kinase activity in fat and skeletal muscle cells → phosphorylation of insulin receptor substrate (IRS)-1 → decreased affinity of IRS-1 for PI3K → decreased expression of GLUT4 channels → decreased cellular glucose uptake
- Numerous genetic and environmental factors
- Pancreatic β cell dysfunction: accumulation of pro-amylin (islet amyloid polypeptide) in the pancreas → decreased endogenous insulin production
- Peripheral insulin resistance
- Progression
- Initially, insulin resistance is compensated by increased insulin and amylin secretion.
- Over the course of the disease, insulin resistance progresses, while insulin secretion capacity declines.
- After a period of impaired glucose tolerance with isolated postprandial hyperglycemia, diabetes manifests with fasting hyperglycemia.
Glucagon in diabetes
- Normal Physiology: Insulin normally exerts a paracrine inhibitory effect on -cells in the pancreatic islets.
- Dysregulation: Lack of insulin inhibition Paradoxical Hyperglucagonemia. t
- Effect: Drives hepatic gluconeogenesis Fasting hyperglycemia.
- DKA Role: Essential for ketogenesis. Glucagon/Insulin ratio disinhibits CPT-1 Fatty Acid oxidation.
Clinical features
Onset
Type 1 DM
- Often sudden
- Diabetic ketoacidosis (DKA) is the first manifestation in approx. one-third of cases.
Type 2 DM
- Typically gradual
- The majority of patients are asymptomatic.
- Some patients may present with a hyperglycemic crisis.
- Elderly patients especially may present in a hyperosmolar hyperglycemic state.
- Occasionally, patients with T2DM present with DKA , which mostly affects black and Hispanic individuals.
- Symptoms of complications may be the first clinical sign of disease.
Common clinical features
- Classic symptoms of hyperglycemia
- Polyuria, which can lead to secondary enuresis and nocturia in children
- Polydipsia
- Polyphagia
- Nonspecific symptoms
- Unexplained weight loss
- Visual disturbances, e.g., blurred vision
- Fatigue
Specific clinical features
Type 2 DM
- Possible cutaneous signs of insulin resistance
- Benign acanthosis nigricans
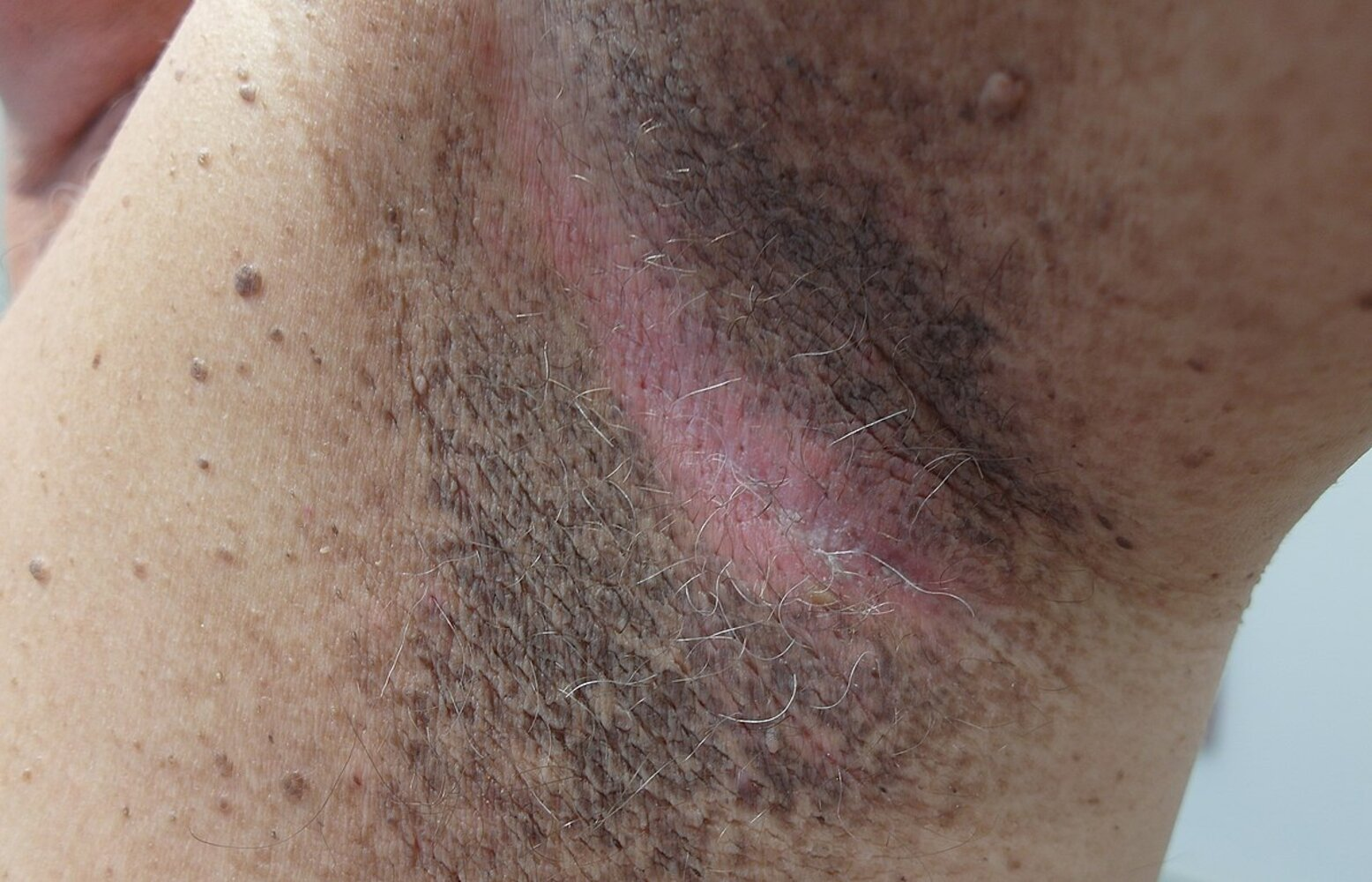
- Acrochordons
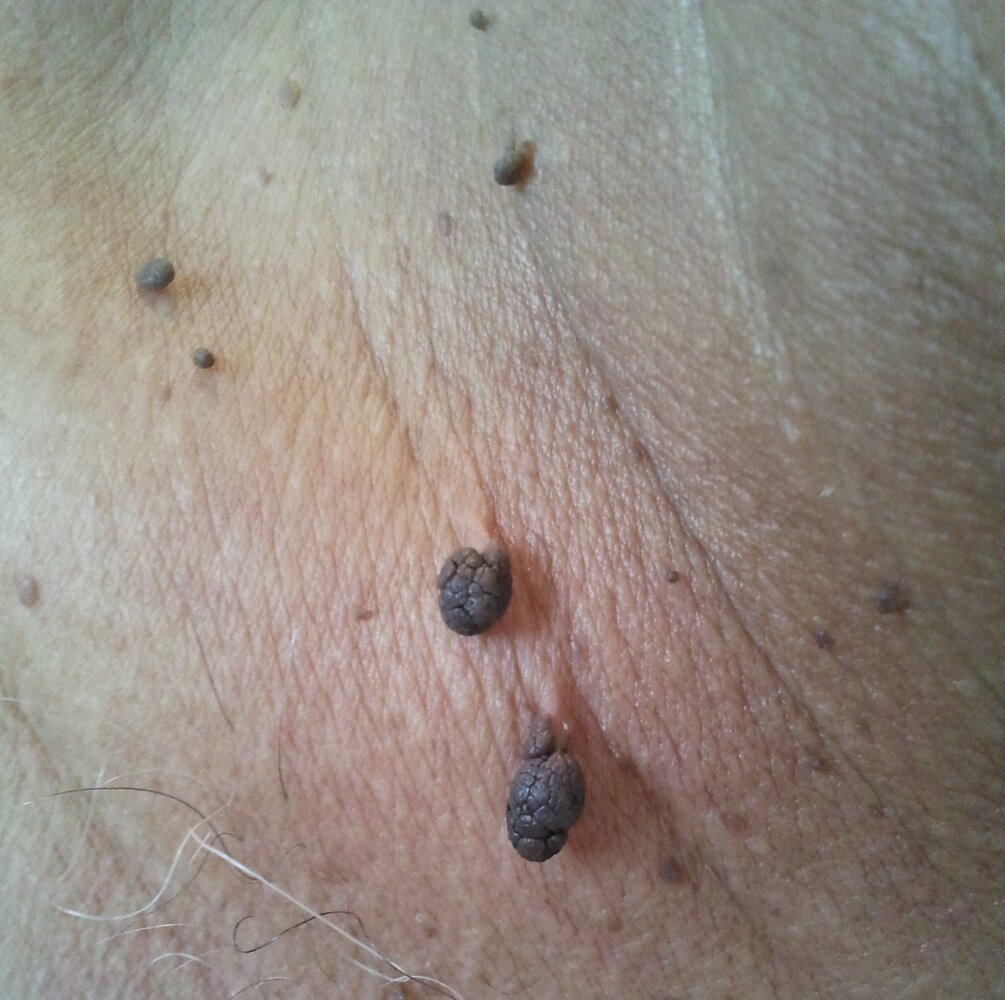
- Benign acanthosis nigricans
Complications
Macrovascular complications of diabetes (atherosclerosis)
- Coronary heart disease (most common cause of death) t
Microvascular disease
- Onset: typically arises 5–10 years after onset of disease
- Pathophysiology: chronic hyperglycemia → nonenzymatic glycation of proteins and lipids → thickening of the basal membrane with progressive function impairment and tissue damage
- Manifestations
- Diabetic nephropathy
- Diabetic retinopathy, glaucoma
- Diabetic neuropathy including diabetic gastroparesis
- Diabetic foot
Tip
Strict glycemic control is crucial in preventing microvascular disease.
Other complications
- Osmotic damage: occurs in tissues with high aldolase reductase activity and low/absent sorbitol dehydrogenase activity (e.g., eyes, peripheral nerves) → diabetic cataracts, neuropathy t
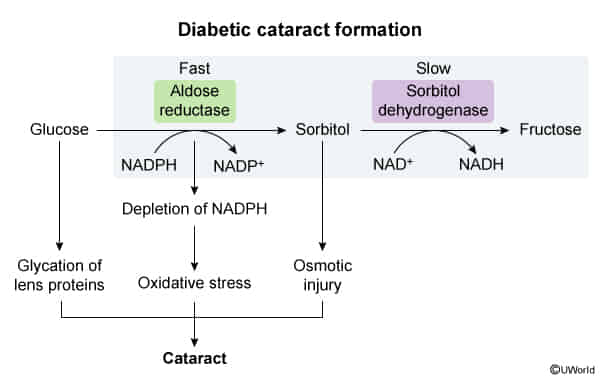
- Pathophysiology (The Polyol Pathway)
- Mechanism: Chronic hyperglycemia saturates normal glycolytic pathways, shunting glucose into the Polyol pathway.
- Key Enzyme: Aldose reductase converts Glucose Sorbitol.
- Accumulation: Sorbitol accumulates because the next enzyme (Sorbitol dehydrogenase, which converts Sorbitol Fructose) is easily overwhelmed or absent in the lens.
- Consequence: Sorbitol is an osmotically active agent.
- Causes water influx into the lens hydropic lens fibers rupture and opacification.
- Also contributes to oxidative stress and non-enzymatic glycosylation of lens proteins.
- Pathophysiology (The Polyol Pathway)
- Necrobiosis lipoidica
- Association: Strong link to Diabetes Mellitus (Type 1 > 2); F > M.
- Presentation: Anterior shins (pretibial); starts as red papules, evolves into yellow-brown waxy/shiny plaques with central atrophy and telangiectasias.
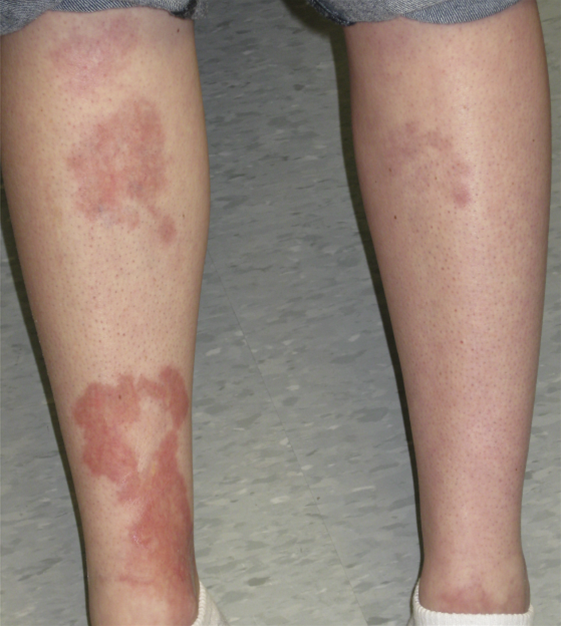
- Diagnosis: Usually clinical; Biopsy shows palisading granulomas (only done if unclear).
- Management:
- Asymptomatic: Observation + trauma protection.
- Ulcerated/Active: Topical/Intralesional Corticosteroids.
- Key Fact: Tight glucose control does NOT improve the condition. Monitor for Squamous Cell Carcinoma in chronic ulcers.
Diagnostics
Mnemonic
Remember HbA1c as 4,5,6,7
| Test | Normal | Prediabetes | Diabetes |
|---|---|---|---|
| HbA1c | <5.7% | 5.7% – 6.4% | ≥6.5% |
| FPG | <100 mg/dL | 100 – 125 mg/dL | ≥126 mg/dL |
| 2-hr OGTT | <140 mg/dL | 140 – 199 mg/dL | ≥200 mg/dL |
| Random | N/A | N/A | ≥200 mg/dL + Symptoms |
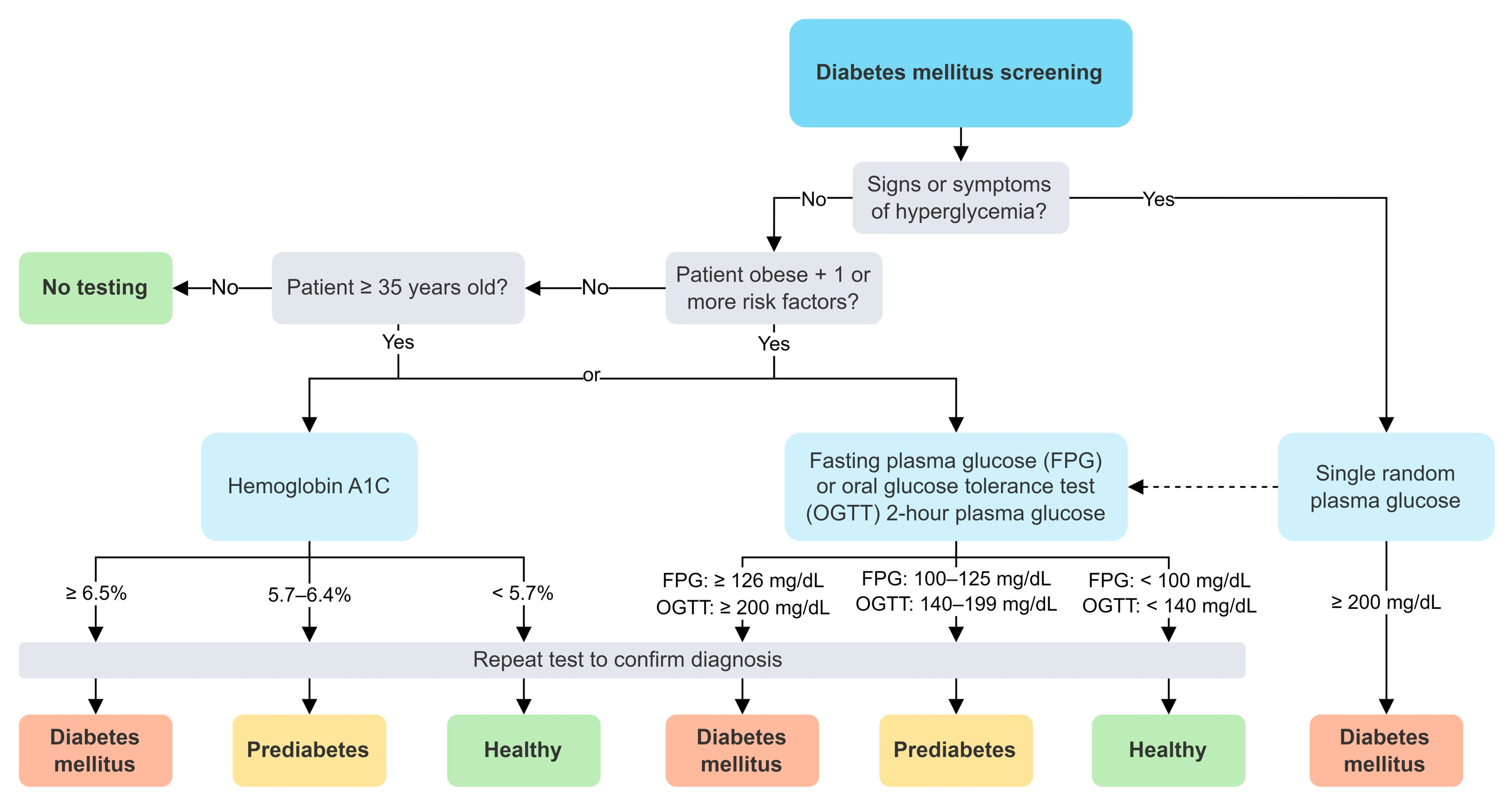
- General Principle: Diagnosis is established using specific blood tests. In asymptomatic individuals, two abnormal test results from the same or different samples are required for confirmation. If a patient has classic symptoms of hyperglycemia (e.g., polyuria, polydipsia, unexplained weight loss) or is in a hyperglycemic crisis, a single random plasma glucose test is sufficient for diagnosis.
- Diagnostic Tests & Criteria:
- Hemoglobin A1c (HbA1c):
- Measures: Average blood glucose over the preceding 2-3 months.
- Normal: <5.7%
- Prediabetes: 5.7% to 6.4%
- Diabetes: ≥6.5%
- Caveats: May be inaccurate in conditions affecting RBC lifespan (e.g., sickle cell disease, hemolytic anemia, recent blood transfusion). Not recommended for diagnosing Type 1 diabetes or in pregnancy.
- Fasting Plasma Glucose (FPG):
- Measures: Blood glucose after a fast of at least 8 hours.
- Normal: <100 mg/dL (5.6 mmol/L)
- Prediabetes: 100 to 125 mg/dL (5.6 to 6.9 mmol/L) - known as Impaired Fasting Glucose (IFG).
- Diabetes: ≥126 mg/dL (7.0 mmol/L)
- Oral Glucose Tolerance Test (OGTT):
- Measures: 2-hour plasma glucose after a 75-gram oral glucose load.
- Normal: <140 mg/dL (7.8 mmol/L)
- Prediabetes: 140 to 199 mg/dL (7.8 to 11.0 mmol/L) - known as Impaired Glucose Tolerance (IGT).
- Diabetes: ≥200 mg/dL (11.1 mmol/L)
- Random Plasma Glucose:
- Measures: Blood glucose at any time of day, without regard to the last meal.
- Diabetes: ≥200 mg/dL (11.1 mmol/L) only if the patient has classic symptoms of hyperglycemia (polyuria, polydipsia, polyphagia, unexplained weight loss) or is in a hyperglycemic crisis (DKA/HHS).
- Hemoglobin A1c (HbA1c):
Diagnostic criteria for diabetes mellitus
Hyperglycemia tests
Tip
In the initial stages, glucose is typically normal when fasting (ie, able to produce some insulin) but elevated after a glycemic load.
- Hemoglobin A1C (HbA1c or A1C): glycated hemoglobin, which reflects the average blood glucose levels of the prior 8–12 weeks
- Can be measured at any time, not required to fast and the results are independent of time of day
- Results may be altered by a variety of conditions or treatments, e.g., sickle cell trait, chronic kidney disease.
- Factors resulting in a falsely high HbA1c
- Increased RBC lifespan: e.g., iron and/or vitamin B12 deficiency, splenectomy, aplastic anemia
- Assay interference: heavy alcohol use
- Factors resulting in a falsely low HbA1c
- Decreased RBC lifespan: e.g., due to acute blood loss, hemoglobinopathies such as sickle cell trait/disease, thalassemia, G6PD deficiency, cirrhosis, hemolytic anemia, splenomegaly, antiretroviral drugs
- Increased erythropoiesis: e.g., due to EPO therapy, reticulocytosis, pregnancy (second and third trimesters), iron supplementation
Additional studies
- C-peptide: can help differentiate between types of diabetes
- ↑ C-peptide levels may indicate insulin resistance and hyperinsulinemia → T2DM
- ↓ C-peptide levels indicate an absolute insulin deficiency → T1DM
Glycemic monitoring for diabetes
Early morning hyperglycemia may be caused by:
| Feature | Dawn Phenomenon | Somogyi Effect |
|---|---|---|
| 3 a.m. Blood Glucose | Normal or High | Low |
| Underlying Cause | Natural hormonal surge | Rebound from hypoglycemia |
| Treatment | Increase evening insulin/meds | Decrease evening insulin or add a snack |
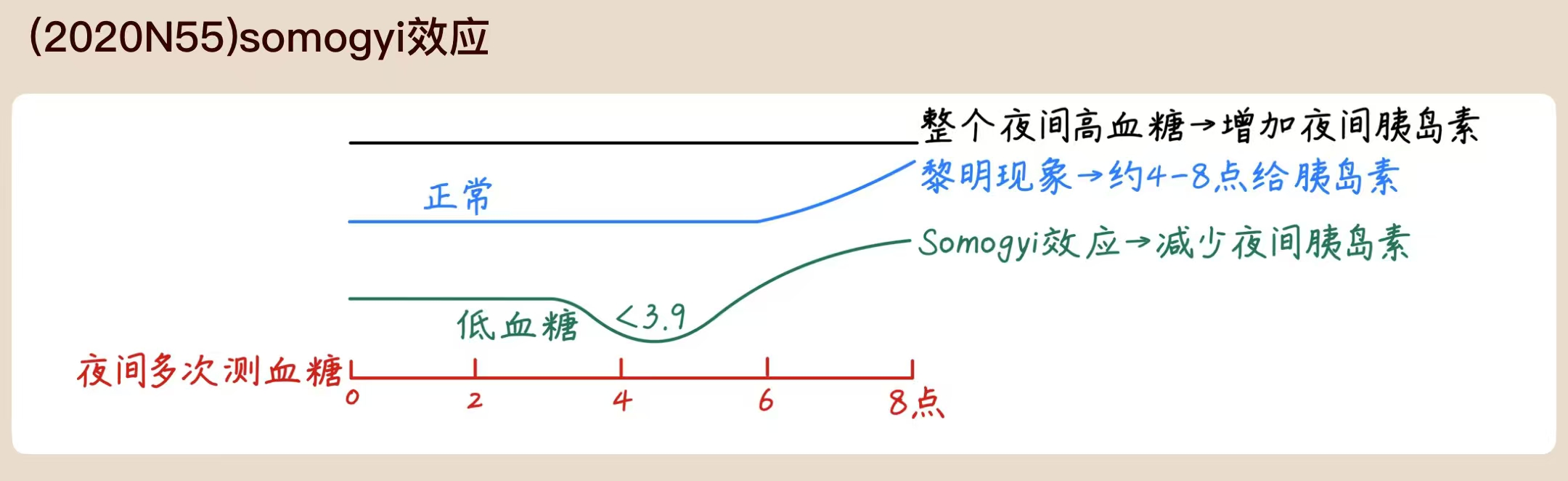
- Dawn phenomenon
- A physiological increase of growth hormone levels in the early morning hours stimulates hepatic gluconeogenesis to prepare for waking, and leads to a subsequent increase in insulin demand. In people with diabetes, insufficient insulin response to this glucose surge leads to hyperglycemia.
- Consider measurement of nocturnal blood glucose levels before initiating insulin therapy.
- Long-acting insulin dose may be given later or increased under careful glycemic control.
- Somogyi effect (widely taught but unproven hypothesis)
- Description: Nocturnal hypoglycemia due to evening insulin injection triggers a counterregulatory secretion of hormones , leading to elevated blood glucose levels in the morning.
- There is no evidence to support the existence of this effect.
Glycemic treatment
Approach
- Guiding Principles (ADA/EASD): Initial therapy is lifestyle modification + Metformin. Subsequent agent selection is based on patient comorbidities (ASCVD, HF, CKD), risk of hypoglycemia, impact on weight, and cost.
- First-Line (Type 2 DM):
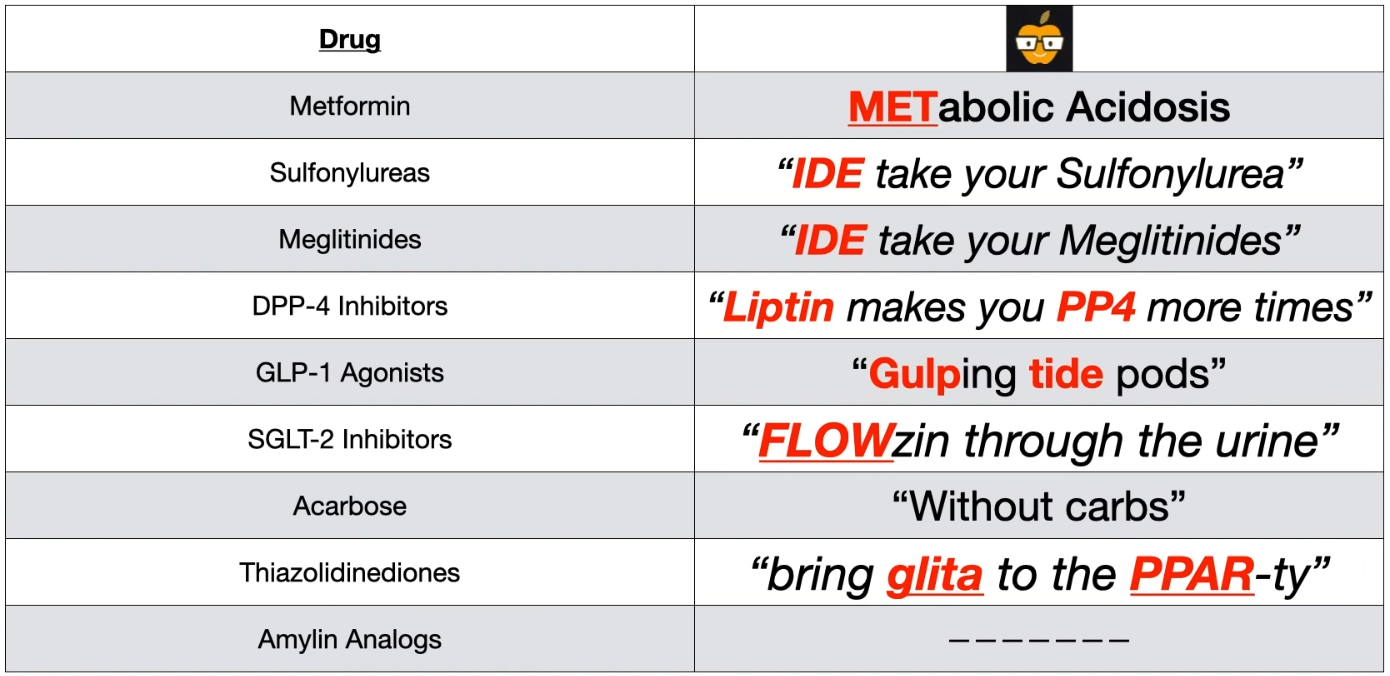
- Metformin:
- MOA: ↓ Hepatic gluconeogenesis, ↑ peripheral insulin sensitivity.
- Benefits: Weight neutral/modest loss, low hypoglycemia risk, proven efficacy.
- SE/CI: GI distress (diarrhea), lactic acidosis (do not use if eGFR <30), B12 deficiency. Hold for IV contrast procedures.
- Metformin:
- Second-Line Selection (after Metformin):
- If patient has ASCVD, HF, or CKD: Choose an agent with proven cardiorenal benefit, regardless of A1c.
- ASCVD Dominant: GLP-1 RA (e.g., Liraglutide, Semaglutide) > SGLT2i (e.g., Empagliflozin).
- HF or CKD: SGLT2i is preferred (reduces HF hospitalizations & CKD progression). GLP-1 RA is a secondary option if SGLT2i is CI/not tolerated.
- If compelling need to minimize hypoglycemia:
- Use DPP-4i, GLP-1 RA, SGLT2i, or TZD. Avoid Sulfonylureas & Insulin.
- If compelling need for weight loss:
- Use GLP-1 RA (most effective) or SGLT2i.
- If cost is a major issue:
- Use Sulfonylurea or TZD. Be mindful of side effect profiles.
- If patient has ASCVD, HF, or CKD: Choose an agent with proven cardiorenal benefit, regardless of A1c.
- If Glucose Control Remains Suboptimal (Third-Line Therapy):
- Action: Add a third agent with a complementary mechanism of action.
- Selection: Re-evaluate and prioritize based on patient comorbidities (ASCVD, HF, CKD), hypoglycemia risk, and weight goals.
- High-Benefit Combo: For patients with ASCVD/HF/CKD, combining Metformin + SGLT2i + GLP-1 RA offers maximal cardiorenal protection and potent glucose lowering.
- Intensification: If triple therapy fails or A1c remains significantly elevated (> 10%), initiate/intensify injectable therapy, typically starting with basal insulin once daily.
| Drug Class | Primary Site of Action | Insulin Secretion | Insulin Sensitivity | Glucagon Secretion | Glucose Production / Absorption | High-Yield Fact |
|---|---|---|---|---|---|---|
| Metformin | Liver, Muscle | ↔ | ↑↑ | ↓ | ↓↓ Hepatic | Lactic acidosis risk |
| Sulfonylureas | Pancreas (β-cell) | ↑↑↑ | ↔ | ↓ | ↔ | Hypoglycemia |
| TZDs / Glitazones | Muscle, Adipose | ↔ | ↑↑↑ | ↔ | ↓ Hepatic | PPAR-γ agonist; CHF risk |
| GLP-1 Agonists | Pancreas, CNS | ↑↑ (glucose-dep) | ↔ / ↑ | ↓↓ | ↓ Gastric Emptying | Weight Loss, CV benefit |
| DPP-4 Inhibitors | (Systemic vs GLP-1) | ↑ (glucose-dep) | ↔ | ↓ | ↔ | Weight Neutral |
| SGLT-2 Inhibitors | Kidney (PCT) | ↔ | ↔ / ↑ | ↔ | Blocks reabsorption | CV/Renal benefit, UTIs |
| α-Glucosidase Inh. | Intestine | ↔ | ↔ | ↔ | ↓ Absorption | Major GI side effects |
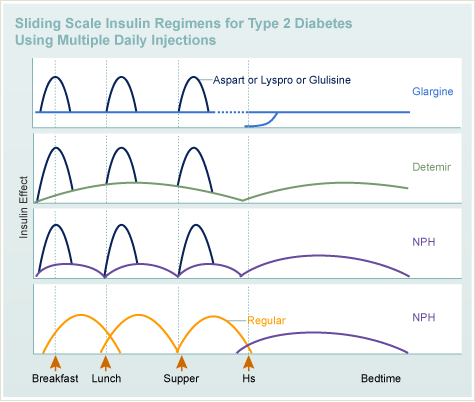
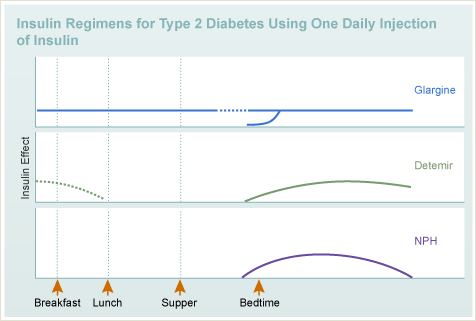
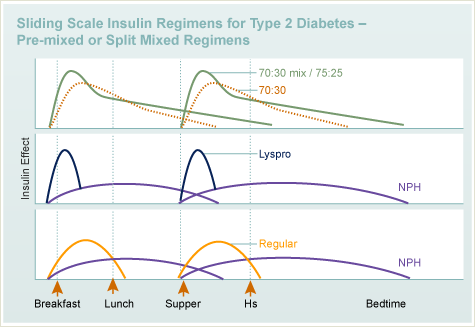
T1DM
Insulin
- Types
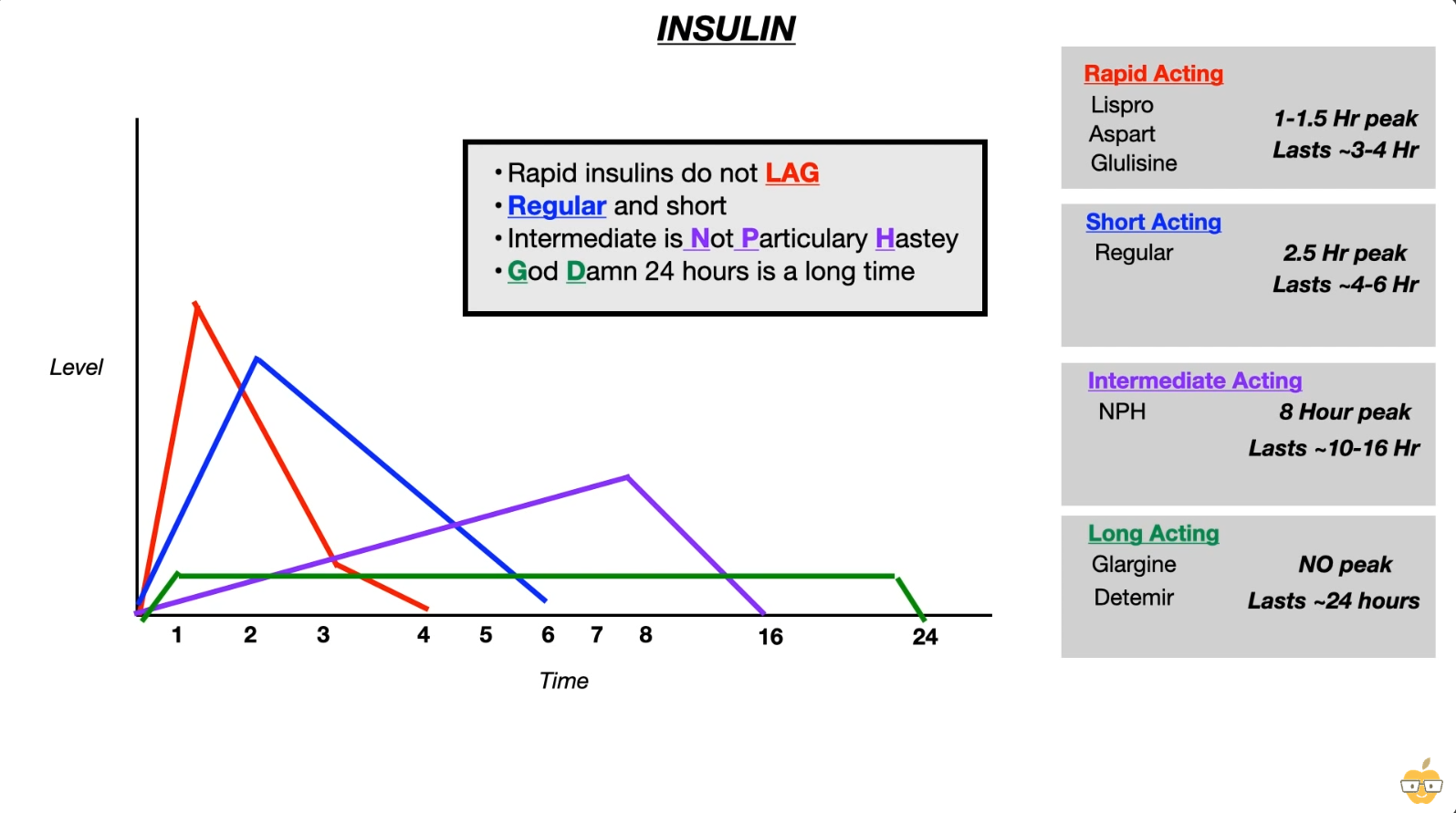
Mnemonic
- Rapid acting
- Lispro: 利索
- Aspart: A-speed
- glulisine: glu利索
- Long acting
- Glargine, detemir: large time to determine
- Rapid Acting
- Drugs: Lispro, Aspart, Glulisine (Remember: “No LAG”).
- Kinetics: Onset ~15 min, Peak 1 hr, Duration 2–4 hrs.
- Use: Postprandial glucose control (inject immediately before meals).
- Short Acting
- Drugs: Regular insulin.
- Kinetics: Onset 30 min, Peak 2–3 hrs, Duration 5–8 hrs.
- Use:
- Postprandial control.
- Only form used IV (Tx for DKA, HHS, Hyperkalemia).
- Intermediate Acting
- Drugs: NPH (Neutral Protamine Hagedorn).
- Kinetics: Onset 2 hrs, Peak 4–12 hrs, Duration ~18 hrs.
- Use: Basal control (often given 2x daily).
- Long Acting
- Drugs: Glargine, Detemir, Degludec.
- Kinetics: No distinct peak (flat profile); Duration ≥ 24 hrs.
- Use: Basal glucose control (once daily).
Tip
- Rapid acting insulin has to be injected before meal, so that its peak action coincides with the rise in blood glucose levels from the food.
- Glargine is a long-acting insulin analogue that forms insoluble complexes. This leads to the formation of microprecipitates at the injection site that then slowly dissolve and are released into the circulation throughout the day.
- Regulation
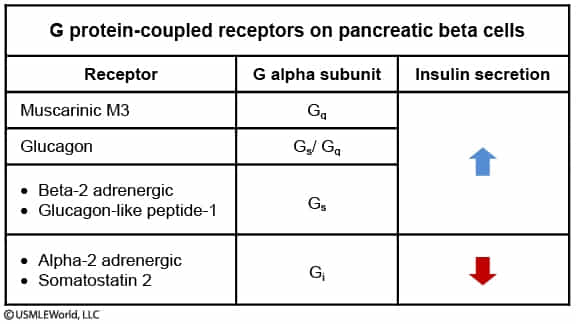
- Sympathetic Nervous System (SNS)
- Net Effect: Inhibition of insulin secretion ( Insulin).
- Physiologic Goal: Mobilize glucose for energy during “fight or flight” (stress, exercise), preventing storage.
- Mechanism:
- Mediated by Catecholamines (Epinephrine, Norepinephrine).
- -adrenergic receptors: Inhibit release (Gi coupled cAMP). This is the predominant effect. t
- -adrenergic receptors: Stimulate release (Gs coupled cAMP), but are clinically masked/overridden by the stronger inhibitory effect.
- Parasympathetic Nervous System (PNS)
- Net Effect: Stimulation of insulin secretion ( Insulin).
- Physiologic Goal: Facilitate nutrient storage during “rest and digest” (e.g., Cephalic phase of digestion).
- Mechanism:
- Mediated by the Vagus nerve via Acetylcholine (ACh).
- Acts on Muscarinic receptors on pancreatic -cells.
- Pathway: Gq coupled IP3/DAG Intracellular Exocytosis of insulin granules.
- Sympathetic Nervous System (SNS)
- Adverse effects
- Weight gain
- Due to physiologic factors (increased glucose uptake and lipid synthesis) and behavioral factors (less dietary adherence and eating snacks when hypoglycemia)
- Should review for changes in diet
- Weight gain
- Regimens
T2DM
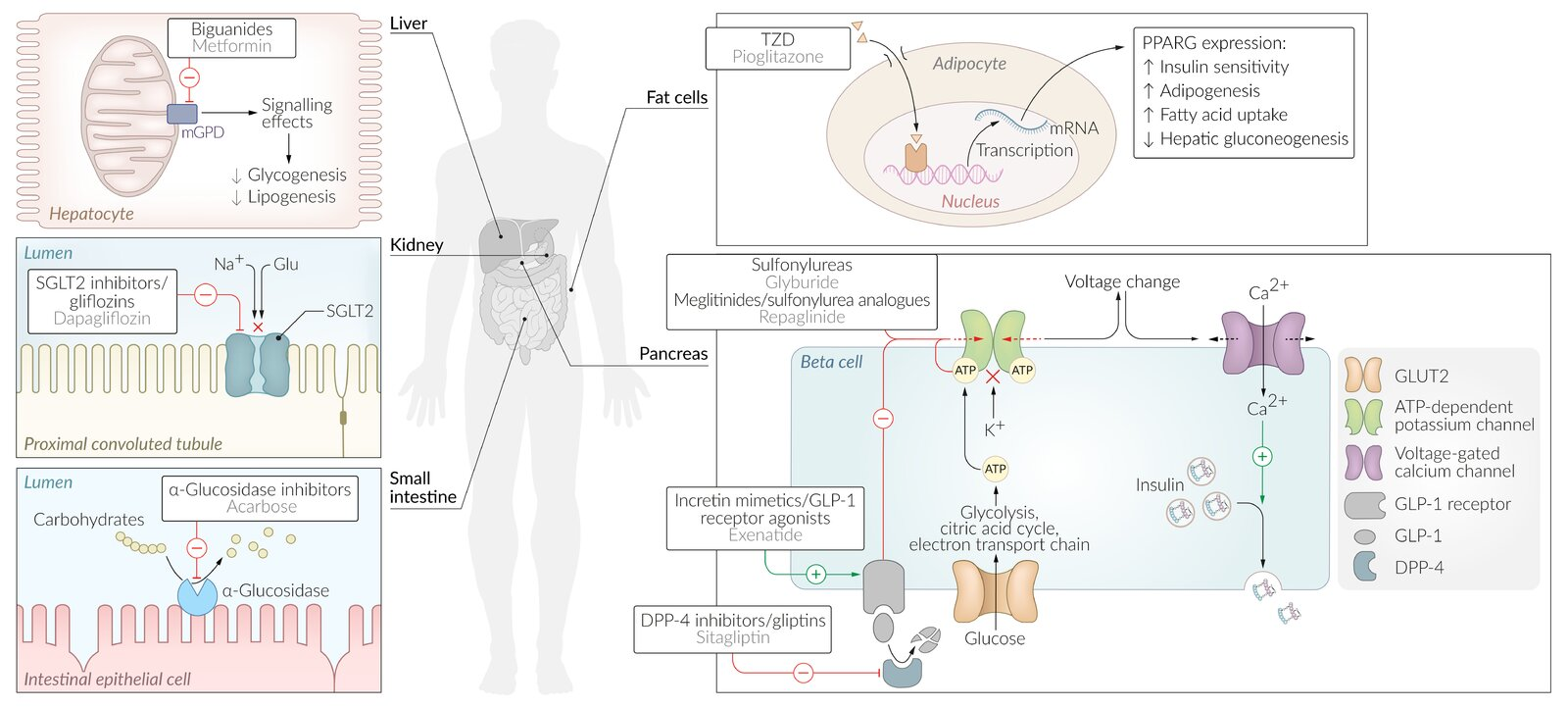
Weight-favorable?
- Weight Loss
- GLP-1 analogs (e.g., Semaglutide, Liraglutide, Exenatide):
- Significant weight loss. t
- Mechanism: Delayed gastric emptying and ↑ satiety.
- SGLT2 inhibitors (e.g., Canagliflozin, Dapagliflozin, Empagliflozin):
- Modest weight loss.
- Mechanism: Glucosuria/caloric loss and osmotic diuresis.
- Metformin (Biguanide):
- Modest weight loss or weight neutral.
- Weight Neutral
- DPP-4 inhibitors (e.g., Sitagliptin, Linagliptin).
- Alpha-glucosidase inhibitors (e.g., Acarbose, Miglitol).
- Weight Gain
- Sulfonylureas (e.g., Glipizide, Glyburide):
- Mechanism: ↑ Insulin secretion (anabolic).
- Thiazolidinediones (TZDs) (e.g., Pioglitazone, Rosiglitazone):
- Mechanism: Fluid retention and adipose tissue differentiation/redistribution.
- Meglitinides (e.g., Repaglinide, Nateglinide).
- Insulin:
- Significant weight gain (anabolic hormone).
Metformin
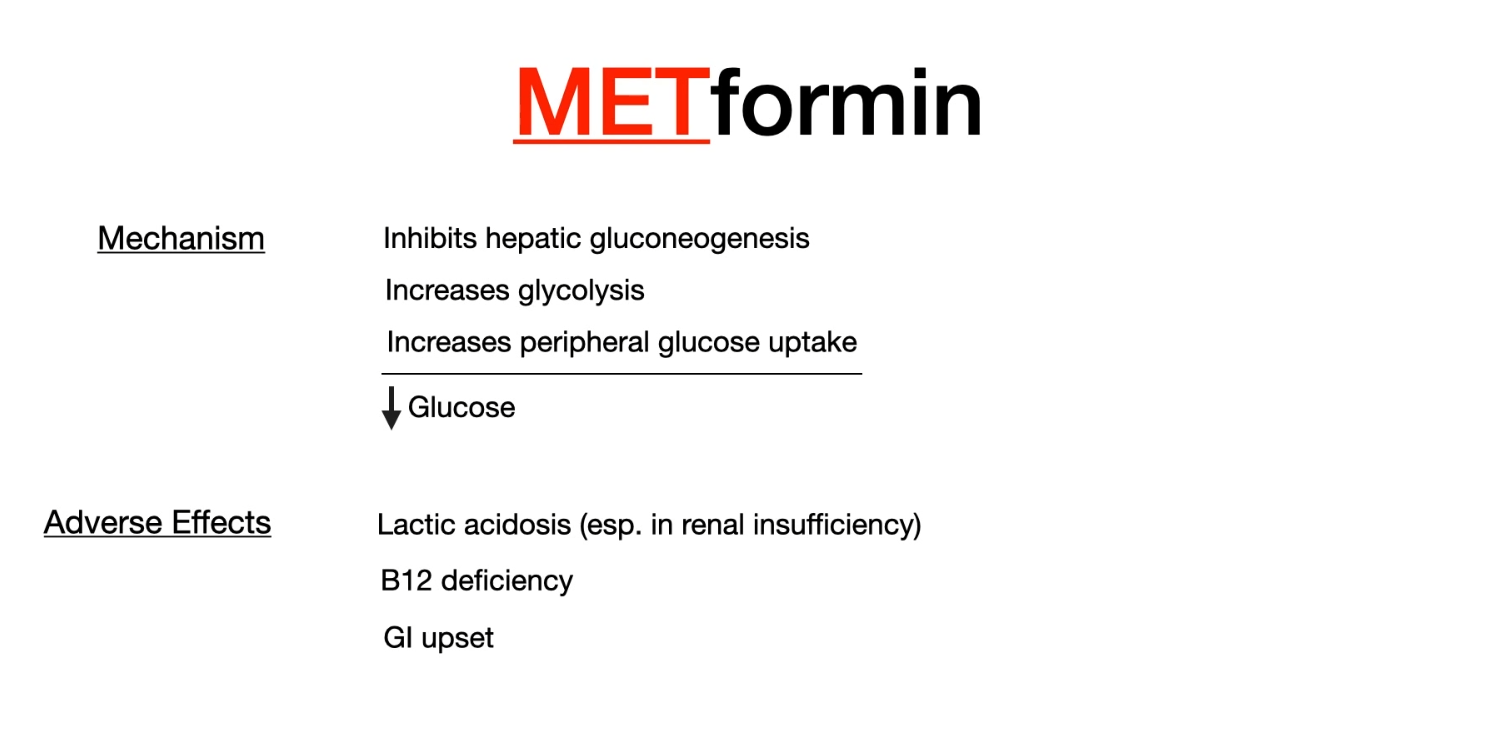
- Mechanism of action: enhances the effect of insulin
- Decreases hepatic gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase.
- Increases glycolysis and peripheral glucose uptake (improves insulin sensitivity).
- Lowers postprandial and fasting blood glucose levels
- Reduces LDL, increases HDL
- Important side effects
- Metformin-associated lactic acidosis
- High-risk groups
- Elderly individuals
- Patients with renal insufficiency or CHF
- So check serum creatinine before initiation of metformin t
- High-risk groups
- Metformin-associated lactic acidosis
- Contraindications
- Renal failure, heart failure (NYHA III and IV), respiratory failure, shock, sepsis
- Decreased renal blood flow, cannot be excreted
- Renal failure, heart failure (NYHA III and IV), respiratory failure, shock, sepsis
Mnemonic
Metformin accumulation will cause metabolic acidosis (lactic acidosis), so stop it before contrast CT scan.
Sulfonylureas

- Drugs: Glipizide, Glyburide, Glimepiride. t
- Mechanism
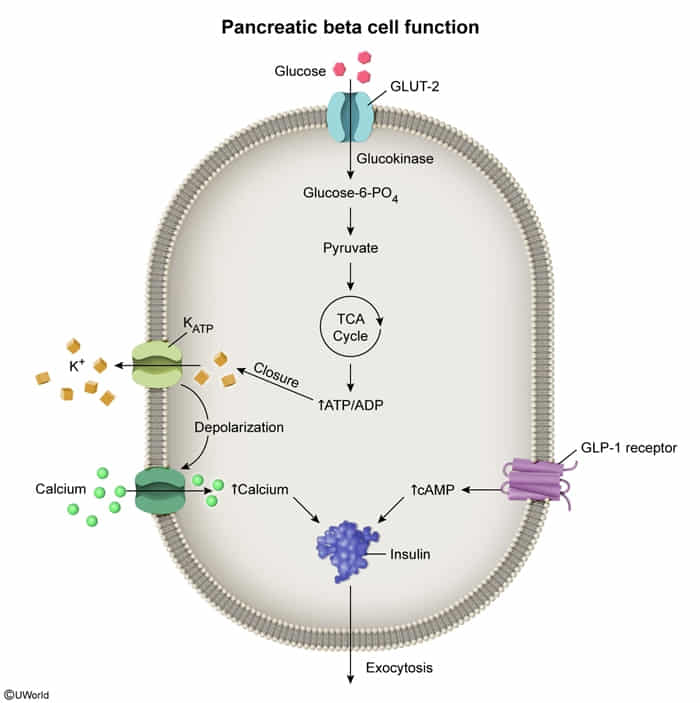
- Close ATP-dependent K+ channels on pancreatic β-cells → depolarization → Ca2+ influx → insulin release.
- Requires functioning β-cells, so not used in T1DM.
- Side effects
- Life-threatening hypoglycemia
- Due to excessive insulin secretion, which occurs independent of blood glucose concentrations (ie, even when blood glucose concentrations are low).
- Increased risk under the following circumstances:
- Simultaneous intake of CYP2C9 inhibitors (e.g., amiodarone, trimethoprim, fluconazole)
- Patients with renal failure
- Because sulfonylureas are excreted via the kidneys, renal failure can result in accumulation. Hypoglycemia due to accumulation of long-acting glyburide may persist for several days, requiring extensive glucose substitution.
- Decreased carbohydrate intake (diets or periods of fasting)
- Elevated glucose utilization (e.g., unaccustomed physical activity)
- Sulfonylurea overdose
- Alcohol intolerance (first-generation agents): disulfiram-like reaction
- Weight gain
- Hematological changes: granulocytopenia, hemolytic anemia
- Life-threatening hypoglycemia
Mnemonic
G (Gee), IDE take your sulfonylurea. The G is because all sulfonylureas start with G. This helps to separate the sulfonylureas from the meglitinides since they also end with IDE
Meglitinides
- Similar to sulfonylureas (bind to K+ channels to ↑ insulin secretion) but with a shorter duration of action. So less risk.

DPP-4 inhibitor and GLP-1 analogs
- GLP-1 Receptor Agonists
- Drugs: Exenatide, Liraglutide, Semaglutide (the “-tides”).
- MoA:
- Mimic endogenous GLP-1 (incretin) → glucose-dependent insulin secretion t, ↓ glucagon secretion, delayed gastric emptying, and increased satiety.
- Although Glucagon-like peptide-1 (GLP-1) and glucagon share similar structure, they perform opposite functions.
- AEs:
- GI side effects are most common (nausea, vomiting, diarrhea).
- Pancreatitis (rare).
- High-Yield Notes:
- Promote significant weight loss.
- Demonstrate major cardiovascular benefits, reducing the risk of major adverse cardiovascular events (MACE).
- Administered via subcutaneous injection (except for an oral form of semaglutide).
- Black Box Warning: Medullary thyroid carcinoma (seen in animal studies).
Tip
The “glucose-dependent” nature of GLP-1 agonists means they potentiate insulin secretion only when blood glucose is elevated, drastically reducing the risk of hypoglycemia compared to the “always-on” mechanism of sulfonylureas.
- DPP-4 Inhibitors
- Drugs: Sitagliptin, Saxagliptin, Linagliptin (the “-gliptins”).
- MoA:
- Inhibit the dipeptidyl peptidase-4 (DPP-4) enzyme, which normally breaks down endogenous incretins (like GLP-1).
- This increases endogenous GLP-1 levels, leading to glucose-dependent insulin release and decreased glucagon.
- AEs:
- Generally well-tolerated.
- May cause nasopharyngitis/upper respiratory infections.
- Possible increased risk of pancreatitis and joint pain (arthralgias).
- High-Yield Notes:
- Weight neutral.
- Lower risk of hypoglycemia compared to sulfonylureas.
- Linagliptin is not cleared renally, so no dose adjustment is needed in renal failure.
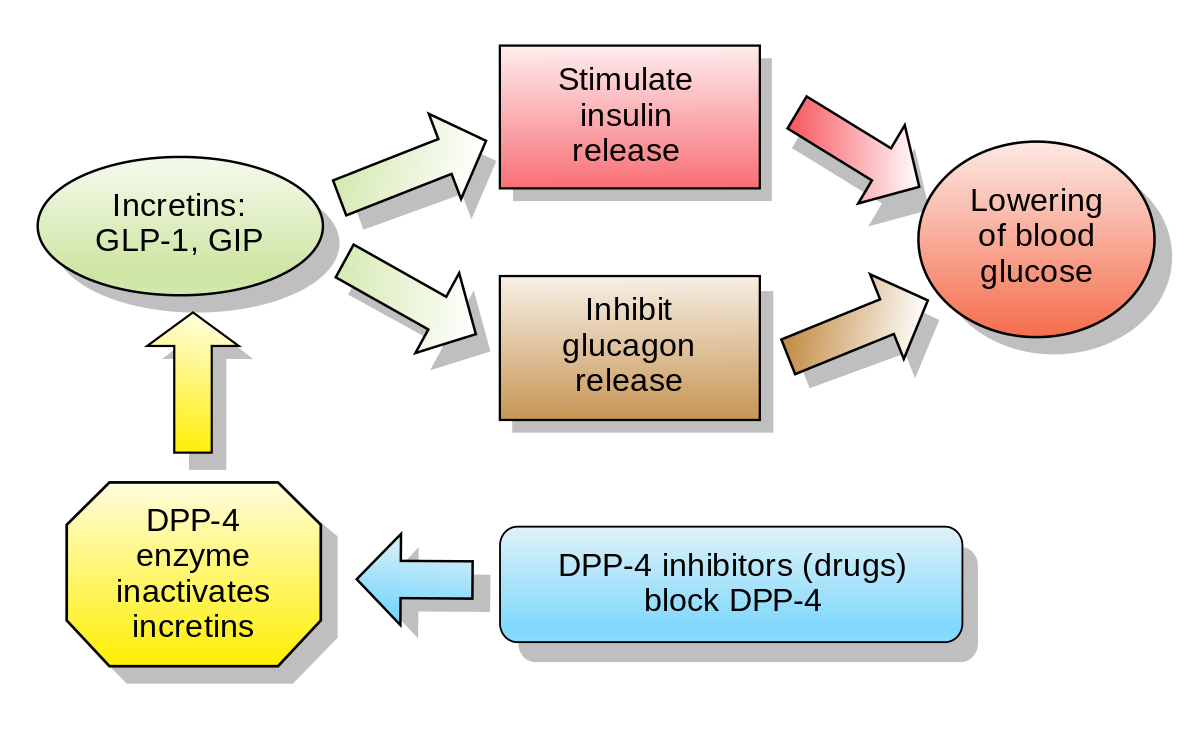

Mnemonic
- Gulfing down tide pods → GLP-1 agonist
- Liptin makes you pee (PP4) more times
SGLT-2 inhibitors
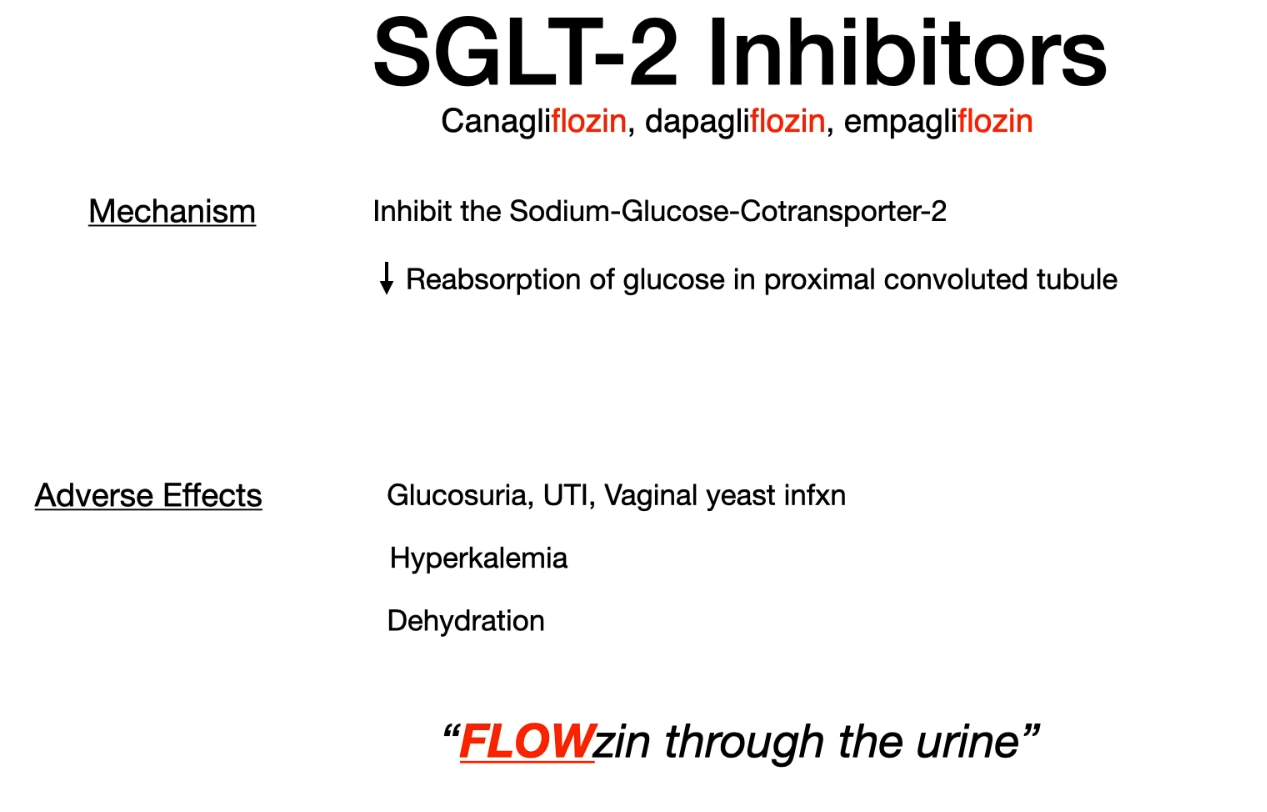
- Sodium-dependent glucose cotransporter (SGLT)
- SGLT1 is responsible for almost all sodium-dependent glucose uptake in the small intestine
- SGLT2 is responsible for almost all sodium-dependent glucose reabsorption in the proximal tubule.
- Contraindications
- Chronic kidney disease: If GFR decreases, drug efficacy decreases and adverse effects increase.
- Because SGLT-2 inhibitors rely on adequate renal glucose filtration to exert their antihyperglycemic effect, they become less effective or ineffective as renal function declines (eg, when estimated glomerular filtration rate (eGFR) falls <30-45 mL/min/1.73 m2). Therefore, checking serum creatinine (used to calculate eGFR) is recommended prior to medication initiation.
- Recurrent urinary tract infections (e.g., in patients with anatomical or functional anomalies of the urinary tract)
- Chronic kidney disease: If GFR decreases, drug efficacy decreases and adverse effects increase.
α-glucosidase inhibitors

Thiazolidinediones (TZD) t

- Mechanism of action
- Binds to PPAR-γ (peroxisome proliferator-activated receptor-gamma) nuclear transcription regulator.
- Upregulates genes for glucose and lipid metabolism (e.g., GLUT4).
- ↑ Insulin sensitivity in peripheral tissue (adipose, muscle). t
- Examples: Pioglitazone, Rosiglitazone.
- Clinical characteristics
- Glycemic efficacy: lowers HbA1c by 1% in 3 months
- Favorable effect on lipid metabolism: ↓ triglyceride, ↓ LDL, ↑ HDL
- No risk of hypoglycemia
- Onset of action is delayed by several weeks.
- because it acts at the level of transcription
- Important side effects
- Fluid retention/Edema: Can cause or exacerbate Congestive Heart Failure (CHF).
- ↑ Risk of bone fractures (osteoporosis)
- Fluid retention and edema
- Weight gain
- Contraindications
- Symptomatic Heart Failure (NYHA Class III or IV).
- Type 1 DM or Diabetic Ketoacidosis (DKA).
- 一心不能二用。一型糖尿病和心衰不能用二酮类药物。
Mnemonic
- glita ← glitter
- Eat in the party will make you gain weight.
Tip
Fibrates activate PPAR-α. See Fibrates
- Amylin analogs (Pramlintide)
- Works with insulin to ↓ glucagon release, ↓ gastric emptying.
Mnemonic
Amylin analogs (Pramlintide) works with insulin.
Screening
- All individuals ≥ 35 years of age
- Patients < 35 years of age who:
- Are overweight or obese AND have ≥ 1 additional risk factor for T2DM
- BMI of ≥ 23 kg/m2 in Asian American individuals or a BMI of ≥ 25 kg/m2 in all other individuals
- Are overweight or obese AND have ≥ 1 additional risk factor for T2DM