Epidemiology
Etiology
- Idiopathic inflammatory autoimmune disorder of unknown etiology
- Risk factors include:
- Genetic disposition: associated with HLA-DR4 and HLA-DR1
- Environmental factors (e.g., smoking)
- Hormonal factors (premenopausal women are at the highest risk, suggesting a predisposing role of female sex hormones)
Pathophysiology
- Core Concept: A systemic autoimmune disease triggered by an environmental factor in a genetically susceptible host, leading to chronic synovial inflammation and joint destruction.
- Initiation
- Genetics: HLA-DR4 is the key association.
- Triggers: Smoking is the most significant environmental risk factor.
- Mechanism: Triggers cause citrullination of self-antigens, creating neoantigens targeted by the immune system.
- Key Immune Components
- TH cells (CD4+): The central drivers. They activate B-cells and macrophages.
- B-cells: Produce autoantibodies:
- Rheumatoid Factor (RF): IgM antibody against the Fc portion of IgG. Low specificity.
- Anti-CCP: Highly specific antibody targeting citrullinated peptides.
- Macrophages: Release key pro-inflammatory cytokines.
- The “Big 3” Cytokines
- TNF-α, IL-1, and IL-6 are the primary mediators of inflammation and joint destruction. (High-yield for pharmacology).
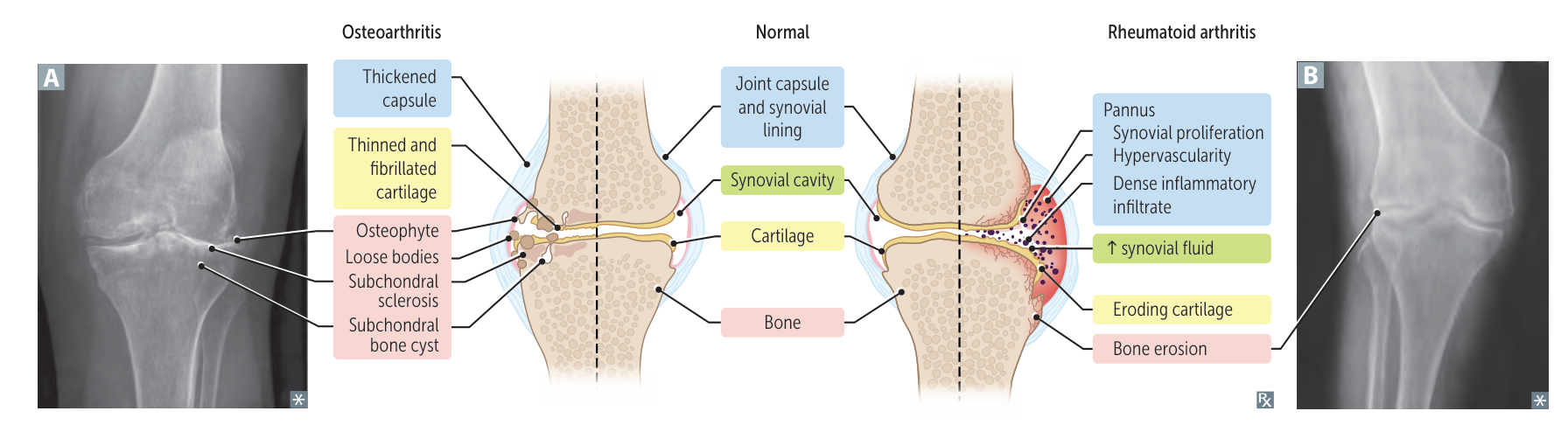
- Joint Destruction Cascade
- Synovitis: Synovial lining becomes inflamed and hypertrophied.
- Pannus Formation: The inflamed synovium transforms into an aggressive a pannus (a mass of synovium, inflammatory cells, and granulation tissue).
- Erosion: The pannus invades and destroys adjacent cartilage (via MMPs) and bone (via RANKL-mediated osteoclast activation).
Clinical features
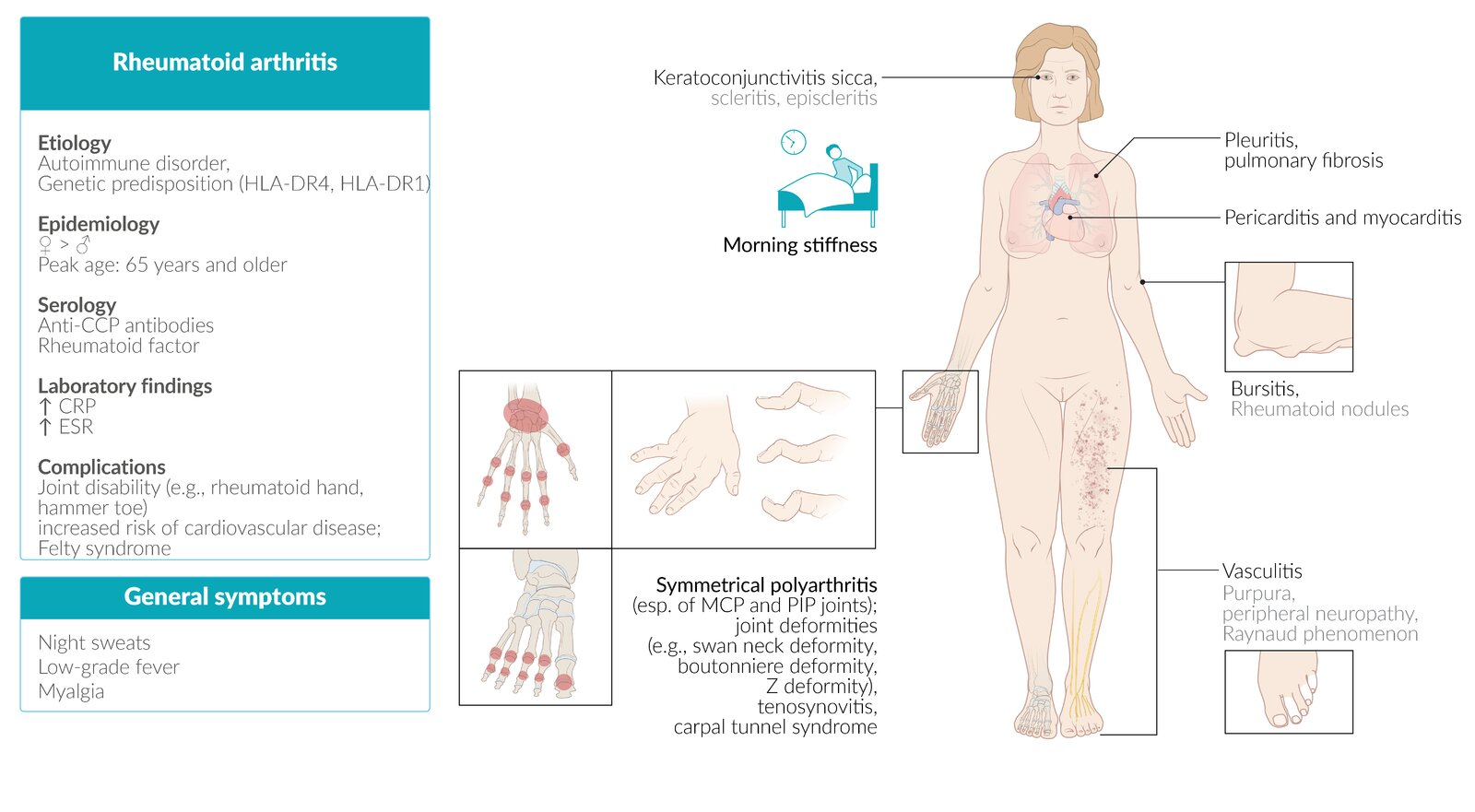
- Articular (Joints):
- Symmetric polyarthritis: Hallmark feature.
- Joints involved: Typically affects small joints of the hands and feet, such as the metacarpophalangeal (MCP), proximal interphalangeal (PIP), and metatarsophalangeal (MTP) joints, as well as the wrists.
- Morning stiffness: Lasts >1 hour and improves with activity (unlike osteoarthritis).
- Spared joints: The distal interphalangeal (DIP) joints are typically spared.
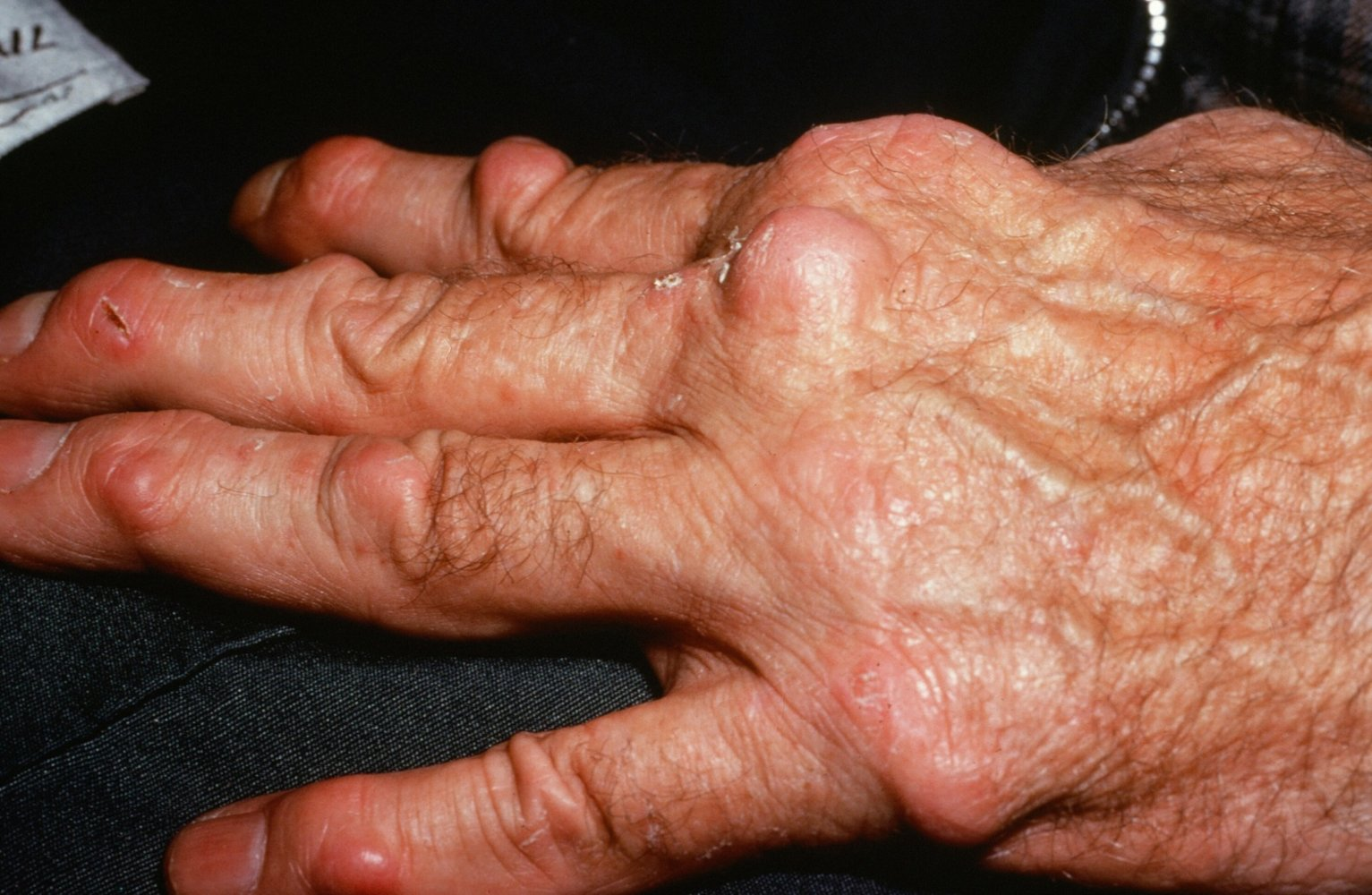
- Deformities (late-stage): Ulnar deviation of fingers, swan-neck deformity (PIP hyperextension, DIP flexion), and Boutonnière deformity (PIP flexion, DIP hyperextension).
- Extra-articular Manifestations:
- Constitutional: Fever, fatigue, weight loss, and malaise are common.
- Rheumatoid Nodules: Most common extra-articular finding; firm, subcutaneous nodules over extensor surfaces or pressure points.
- Pulmonary: Interstitial lung disease, pleuritis, and pulmonary nodules.
- Cardiovascular: Increased risk of premature atherosclerosis and coronary artery disease; pericarditis.
- Hematologic: Anemia of chronic disease, thrombocytosis.
- Ocular: Scleritis, episcleritis, and secondary Sjögren’s syndrome.
Subtypes and variants
Atlantoaxial subluxation (Vertebral subluxation)
- Definition: a potentially life-threatening complication caused by the inflammatory destruction of the ligaments affecting the atlantoaxial joint and the intervertebral joints
- Clinical features
- Pain and stiffness of the neck (typically early-morning neck pain at rest)
- Head tilt
- Neurological deficits
- Cervical radiculopathy with peripheral paresthesias of the upper limb
- In some cases, symptoms of high spinal cord compression
- Slowly progressive spastic quadriparesis
- Hyperreflexia or positive Babinski reflex
- Respiratory insufficiency
- Diagnostics
- Extension and flexion x-rays of the cervical spine
- MRI
Warning
Endotracheal intubation can acutely worsen the subluxation and cause compression of the spinal cord and/or vertebral arteries.
Diagnostics
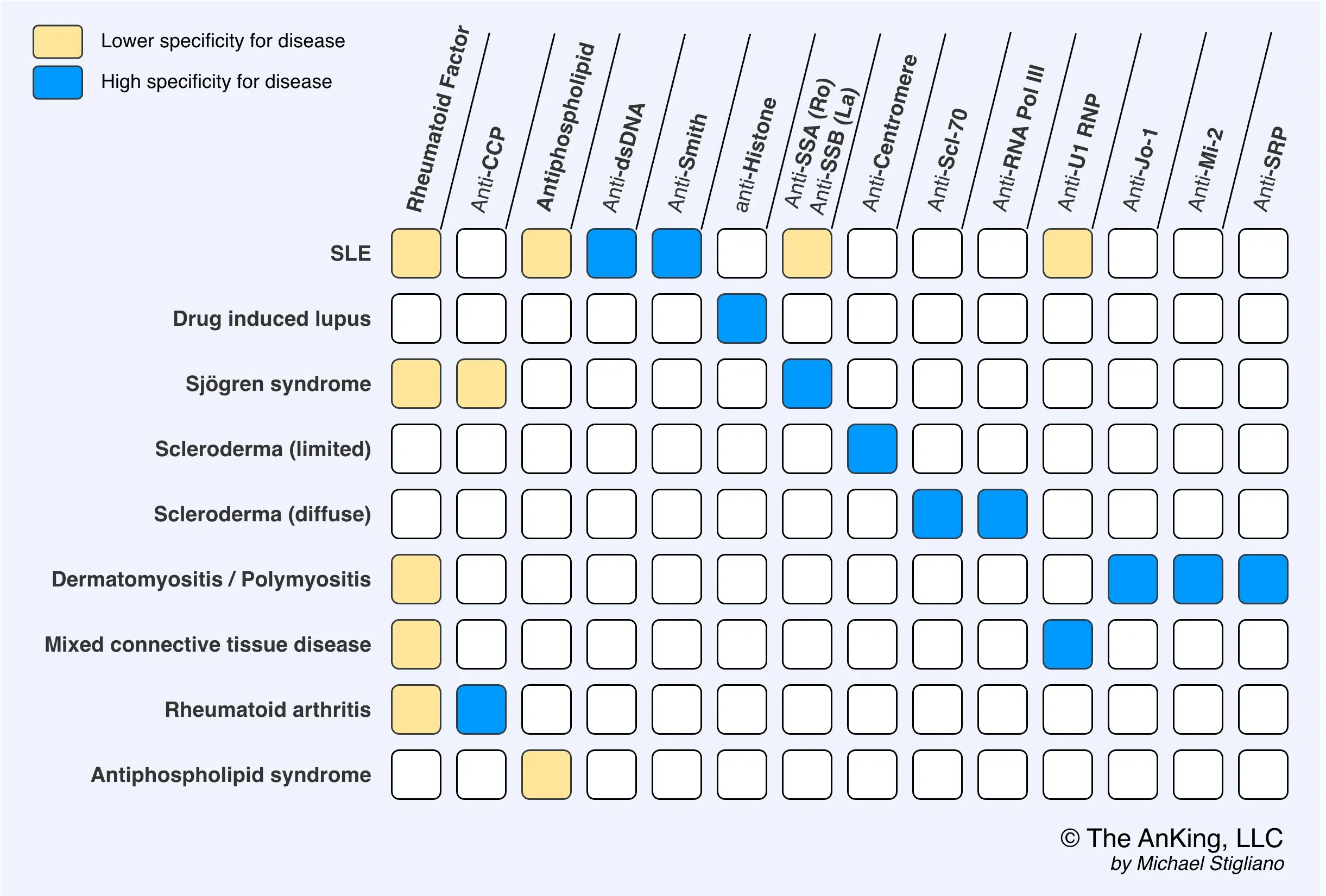
- Anticitrullinated peptide antibodies (ACPA), e.g., anticyclic citrullinated peptide (anti-CCP)
- Tissue inflammation causes arginine residues in proteins such as vimentin to be enzymatically converted into citrulline through a process called citrullination. This alters the shape of the proteins, which can then serve as neoantigens that generate an immune response.
- Rheumatoid factor (RF): IgM autoantibodies against the Fc region of IgG antibodies t
Differential diagnostics
- Parvovirus B19 will presents with similar symptoms, except for rash, normal ESR, and acute onset
- See Differential diagnosis
Osteoarthritis (OA) vs Rheumatoid Arthritis (RA)
Feature Osteoarthritis (OA) Rheumatoid Arthritis (RA) Mechanism Mechanical “Wear & Tear” Autoimmune (Pannus) Stiffness < 30 min (Worse w/ use) > 1 hr (Better w/ use) Symmetry Asymmetric Symmetric Key Joints DIP (Heberden), PIP, Knees MCP, PIP, Wrist (Spares DIP) Labs Normal +Anti-CCP (Specific), +RF, ↑ ESR X-ray Osteophytes, Sclerosis Marginal Erosions, Osteopenia Treatment NSAIDs, Acetaminophen DMARDs (Methotrexate) 1. Stiffness
- OA (<30m): “Gelling.” Synovial fluid gets thick at rest. Movement quickly warms and lubricates it.
- RA (>1h): Edema. Inflammatory fluid pools during sleep. Takes time to mechanically pump/drain the boggy joint.
2. Pain
- OA (Worse w/ use): Mechanical. Bone-on-bone friction compresses exposed nerve endings.
- RA (Better w/ use): Washout. Movement flushes out stagnant inflammatory cytokines and fluid, relieving pressure.
3. Pathology
Link to original
- OA (Osteophytes): Construction. Bone grows spurs to widen surface area and stabilize the failing joint.
- RA (Erosions): Destruction. Pannus (granulation tissue) releases enzymes/RANKL that eat into the bone.
Treatment
Acute anti-inflammatory treatment
- Glucocorticoids
- Systemic prednisone
- Longer-term therapy: Only use in patients with highly active RA
- Systemic prednisone
- NSAIDs and selective COX-2 inhibitors: relieve symptoms, but do not improve the prognosis
Long-term pharmacological treatment
Disease-modifying antirheumatic drugs (DMARDs)
- Methotrexate (MTX): first-line treatment in patients with moderate to high disease activity
- To minimize adverse effects, administer folic acid.
- Hydroxychloroquine: Consider in patients with low disease activity.
- Decreases complement-dependent antigen-antibody reactions
Biologic DMARDs
- Indication: persistent moderate or severe disease activity after 3 months of conventional DMARD therapy
- Agents
- TNF-α inhibitors: e.g., adalimumab, infliximab, etanercept
- See Etanercept
- TNF-α inhibitors: e.g., adalimumab, infliximab, etanercept
Complications
- AA amyloidosis (secondary amyloidosis)
- Septic arthritis