- Cells involved
- Osteoclasts: degrade bone tissue by secreting collagenase and H+
- Regulation
- RANKL: Stimulates differentiation, fusion, activation, and prevents apoptosis (via binding to RANK).
- OPG (Osteoprotegerin): Inhibits activity (by blocking RANKL).
- M-CSF: Stimulates proliferation and differentiation of precursor cells.
- Lack of Mechanical Load: Stimulates activity (leading to decreased bone mass).
- PTH (High Levels): Stimulates activation.
- Estrogen: Inhibits activity (stimulates apoptosis).
- Vitamin D: Stimulates differentiation.
- Bisphosphonates: Inhibit activity.
- Glucocorticoids: Stimulate activity (increase lifespan).
- IL-1: Leads to an increase in RANK ligand signaling and subsequent osteoclast-mediated bone resorption
- Regulation
- Osteoblasts
- Build bone tissue by secreting type I collagen
- Activity assessed by an increase in bone ALP, osteocalcin, and type I procollagen propeptides
- Regulation
- Mechanical Load: Stimulates activity (leading to increased bone mass).
- Growth Factors (Released by Osteoclasts): Stimulates activity.
- PTH (Low Levels): Stimulates bone formation.
- Estrogen: Stimulates activity/survival (inhibits apoptosis).
- Vitamin D: Stimulates differentiation and activation.
- Osteoclasts: degrade bone tissue by secreting collagenase and H+
Mnemonic
Blasts Build, Clasts Crumble.
Bone remodeling in cortical bone
Degradation
- Osteoclasts organize in a basic multicellular unit (BMU) and excavate a tunnel in the cortical bone.
- Connective tissue vessels and unmyelinated nerves grow in the tunnel.
Formation
- Osteoclasts are followed by osteoblasts → deposition of the first osteoid layer in the tunnel
- Additional osteoblasts follow and deposit osteoid onto the first osteoid layer → osteoblasts of the first layer are walled in → osteoblasts become osteocytes
- Osteocyte function relies on the presence of gap junctions that connect the cytoplasmic processes between osteocytes. These junctions facilitate cell-to-cell communication, allowing intracellular signals (eg, calcium, cyclic AMP) to propagate to neighboring cells.
- The deposition process is repeated until the tunnel is almost full → central Haversian canal remains open
- The innermost (i.e., last) generation of osteoblasts is no longer walled in → cells return to their resting state and form the endosteum
Mineralization: occurs successively
- Osteoblasts secrete collagen and vesicles into the extracellular matrix.
- Vesicles contain enzymes (e.g., alkaline phosphatase), which increase local phosphate levels (e.g., by cleavage of pyrophosphate).
- Calcium-binding molecules in the vesicles most likely serve as a focal point.
- Initial formation of hydroxyapatite crystals around the focal point in the vesicles
- Independent growth of the crystals until penetration of the vesicle membrane
- Release of crystals in the extracellular matrix
- Growth of crystals in the extracellular matrix and accumulation of collagen fibrils
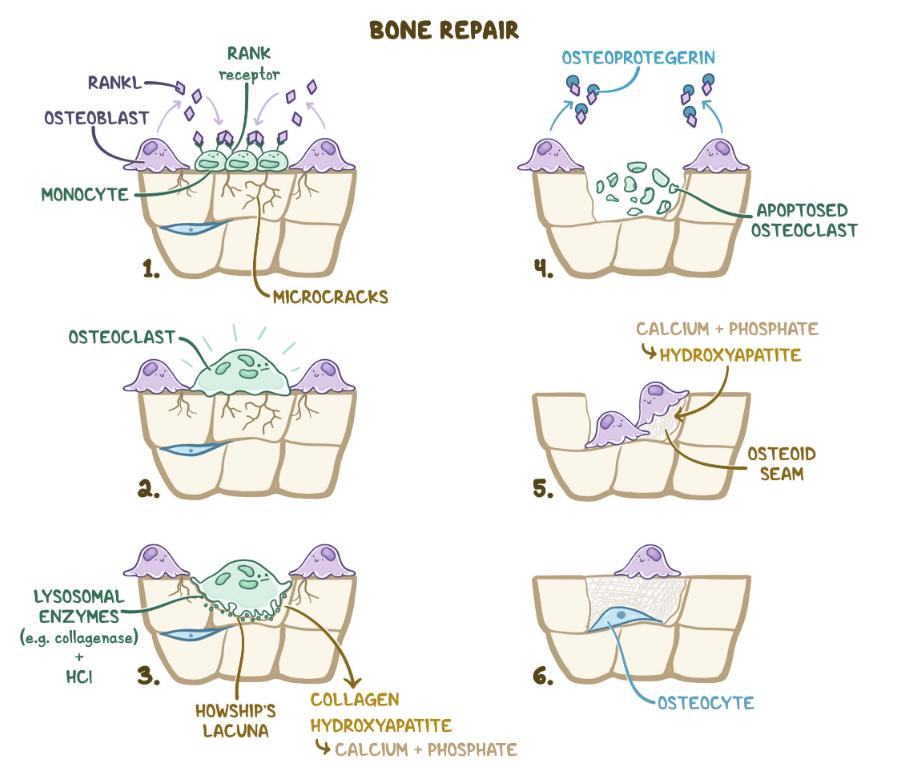
Regulation of bone remodeling
- RANK (receptor activator of nuclear factor κB): receptor on osteoclasts and osteoclast precursors, for interaction with osteoblasts
- RANKL (receptor activator of nuclear factor κB ligand)
- Membrane-bound protein of osteoblasts that stimulates osteoclasts by interacting with RANK
- Ensures fusion and differentiation into activated osteoclasts and prevents their apoptosis
- Osteoprotegerin (OPG)
- A regulatory protein secreted by osteoblasts that binds RANKL
- Inhibits RANK-RANKL interaction, leading to decreased osteoclast activity
- Mechanical load (Wolff’s law)
- Load on the bone leads to increased bone mass.
- Absence of load (e.g., due to being confined in bed) results in decreased bone mass.
- Sensed by osteocytes via extracellular attachments (eg, integrins) to the canalicular walls, resulting in the release of mediators—such as soluble receptor activator of nuclear factor-kappa B (RANKL) and sclerostin—that orchestrate bone remodeling
- Hormones
- PTH effects
- PTH receptors are on osteocytes & osteoblasts, not osteoclasts. PTH controls osteoclasts indirectly.
- 1. Continuous/Sustained High Levels (Catabolic):
- Seen in hyperparathyroidism.
- Mechanism: PTH acts on osteoblasts to ↑ RANK-L and ↓ OPG.
- Result: Increased osteoclast activation → bone resorption → ↑ serum Ca2+.
- Net Effect: Bone breakdown.
- 2. Intermittent/Pulsatile Low Levels (Anabolic):
- Physiologic effect.
- Mechanism: Directly stimulates osteoblast differentiation and activity.
- Result: Increased bone formation and mass.
- Net Effect: Bone growth.
- Clinical Application: Teriparatide (recombinant PTH) for osteoporosis.
- Estrogen effects
- Inhibits apoptosis of osteoblasts, leading to increased bone formation
- Stimulates apoptosis of osteoclasts, leading to decreased bone resorption
- Stimulates closure of the epiphyseal plate in puberty
- Estrogen deficiency (e.g., postmenopausal or after bilateral oophorectomy) leads to increased bone resorption, which can result in osteoporosis.
- Thyroid hormone
- In long-standing hyperthyroidism, T3 stimulates osteoclast differentiation, increased bone resorption, and release of calcium.
- PTH effects
Bone composition
| Feature | Diaphysis (Shaft) | Epiphysis (End) |
|---|---|---|
| Outer Layer | Thick Cortical Bone | Thin shell of Cortical Bone |
| Interior | Hollow Medullary Cavity with Yellow Marrow | Network of Cancellous Bone with Red Marrow |
| Covering | Periosteum | Articular Cartilage (at joint surface), Periosteum elsewhere |
| High-Yield Lesion | Ewing Sarcoma | Giant Cell Tumor |