Overview
-
Large and Irregular Nuclei:
- Appearance: Nuclei are often much larger than normal, with irregular shapes, and take up a greater portion of the cell (high nuclear-to-cytoplasmic ratio). They may appear darker than normal (hyperchromasia).
- Mechanism: Caused by increased DNA content due to errors in cell division and ongoing mutations, leading to genomic instability and altered nuclear structure.
-
Prominent and Irregular Nucleoli:
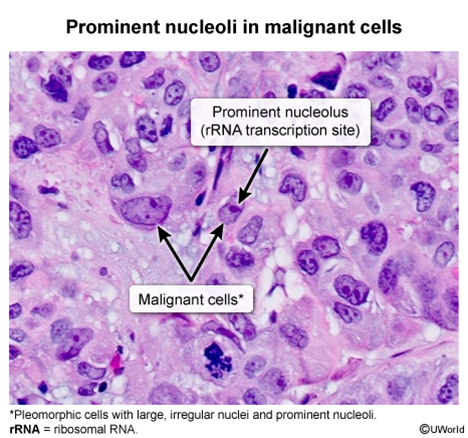
- Appearance: The nucleoli (structures within the nucleus) are often enlarged, more numerous, and irregularly shaped.
- Mechanism: Reflects increased ribosome production needed for the high protein synthesis rates required by rapidly dividing cells.
-
Variation in Cell Size and Shape (Pleomorphism):
- Appearance: Cancer cells within a tumor often vary significantly in their overall size and shape, unlike uniform normal cells.
- Mechanism: Results from ongoing genetic mutations and a breakdown of normal cellular structural controls.
-
Loss of Differentiation (Anaplasia):
- Appearance: Cells lose the specialized features of their normal counterparts and appear more primitive.
- Mechanism: Genetic changes disrupt the normal developmental programs that control cell specialization.
-
Disorganized Arrangement & Loss of Polarity:
- Appearance: Cells lose their normal orderly arrangement within tissues and their typical orientation.
- Mechanism: Caused by alterations in cell adhesion molecules and the proteins that maintain tissue structure and cell orientation, allowing for chaotic growth.
-
Increased and Abnormal Cell Division (Mitoses):
- Appearance: More cells are seen actively dividing, and these divisions may be abnormal (e.g., tripolar spindles).
- Mechanism: Cancer cells bypass normal cell cycle controls due to mutations in regulatory genes, leading to uncontrolled and often faulty cell division.
-
Invasive Growth (Implied Feature):
- Appearance: While not a single cell feature, evidence of cells infiltrating surrounding normal tissues is a hallmark.
- Mechanism: Cancer cells gain the ability to break down tissue barriers and migrate, driven by changes in adhesion, motility, and enzyme production.
Classic examples
- Glioblastoma Multiforme (GBM):
- Pseudopalisading necrosis: Tumor cells line up around areas of necrosis.
- Endothelial proliferation.
- GFAP positive.
- Meningioma:
- Whorled pattern of cells.
- Psammoma bodies: Laminated calcifications.
- Medulloblastoma:
- Small, round, blue cells.
- Homer-Wright rosettes: Circular grouping of tumor cells around a central fibrillary space.
- Pilocytic Astrocytoma:
- Rosenthal fibers: Eosinophilic, corkscrew-shaped glial filaments.
- Cystic lesion with a mural nodule.
- Oligodendroglioma:
- “Fried egg” appearance: Cells with round nuclei, clear cytoplasm, and distinct cell borders.
- “Chicken-wire” capillary pattern.
- Schwannoma:
- Antoni A areas: Densely cellular areas with Verocay bodies (palisading nuclei around an acellular zone).
- Antoni B areas: Less cellular, myxoid areas.
- S-100 positive.
- Hodgkin Lymphoma (Nodular Sclerosing type):
- Reed-Sternberg cells: Large, multinucleated or bilobed (“owl-eye”) cells with prominent eosinophilic nucleoli.
- Lacunar cells: Variant of Reed-Sternberg cells in a clear space (lacuna).
- Fibrous bands dividing lymphoid tissue into nodules.
- Burkitt Lymphoma:
- “Starry sky” appearance: Sheets of lymphocytes (dark sky) interspersed with benign macrophages containing apoptotic bodies (stars).
- Associated with t(8;14) translocation (c-myc).
- Multiple Myeloma:
- Clock-face chromatin in plasma cells (eccentric nucleus, basophilic cytoplasm, perinuclear pale zone - Golgi).
- Rouleaux formation of RBCs (not a tumor cell feature but often seen).
- Papillary Thyroid Cancer:
- “Orphan Annie eye” nuclei: Cells with empty-appearing nuclei due to finely dispersed chromatin and nuclear grooves.
- Psammoma bodies.
- Papillary architecture.
- Medullary Thyroid Cancer:
- Nests or sheets of polygonal or spindle cells.
- Amyloid deposits in the stroma (Congo red positive with apple-green birefringence).
- Small Cell Lung Cancer:
- Small, dark blue cells with scant cytoplasm (Kulchitsky cells).
- Nuclear molding, high mitotic rate.
- Neuroendocrine origin (Chromogranin A, synaptophysin positive).
- Squamous Cell Carcinoma (general):
- Keratin pearls: Concentric layers of squamous cells with keratinization.
- Intercellular bridges.
- Adenocarcinoma (general):
- Gland formation (acini, tubules).
- Mucin production (may see signet ring cells if intracellular mucin displaces nucleus).
- Renal Cell Carcinoma (Clear Cell type):
- Cells with abundant clear cytoplasm (dissolved glycogen and lipids).
- Often arranged in nests with a rich vascular network.
- Wilms Tumor (Nephroblastoma):
- Triphasic pattern: Blastemal (small, round, blue cells), stromal, and epithelial (abortive tubules/glomeruli) components.
- Basal Cell Carcinoma (Skin):
- Nests of basaloid cells with peripheral palisading of nuclei.
- Stromal retraction.
- Melanoma:
- Atypical melanocytes with large, irregular nuclei, prominent nucleoli, and often melanin pigment.
- Nests or individual cells invading the epidermis and/or dermis.
- S-100, HMB-45, Melan-A positive.
- Colorectal Adenocarcinoma (often from adenomatous polyp):
- Dysplastic glandular structures.
- May show “dirty necrosis” in the glandular lumen.
- Serous Cystadenocarcinoma (Ovary):
- Psammoma bodies are common.
- Papillary formations.
- Endometrial Carcinoma (Endometrioid type):
- Back-to-back glands with little intervening stroma.
- Prostate Adenocarcinoma:
- Crowded glands lacking basal cell layer.
- Prominent nucleoli.
- Ewing Sarcoma:
- Small, round, blue tumor cells.
- Sheets of uniform cells.
- Associated with t(11;22) translocation.
- Osteosarcoma:
- Malignant osteoblasts producing osteoid (immature bone).
- Pleomorphic cells.