Alcohol metabolism
Key Point
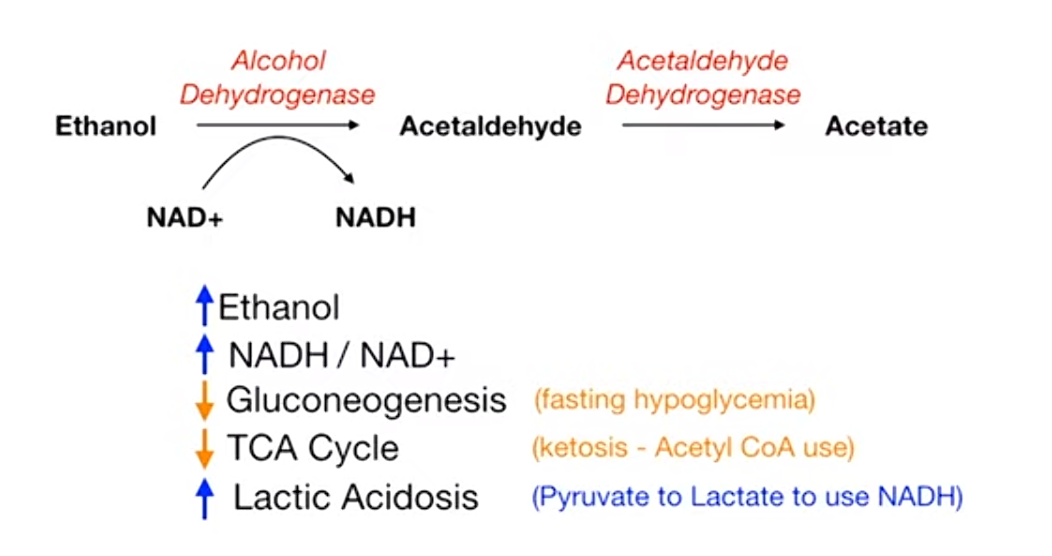
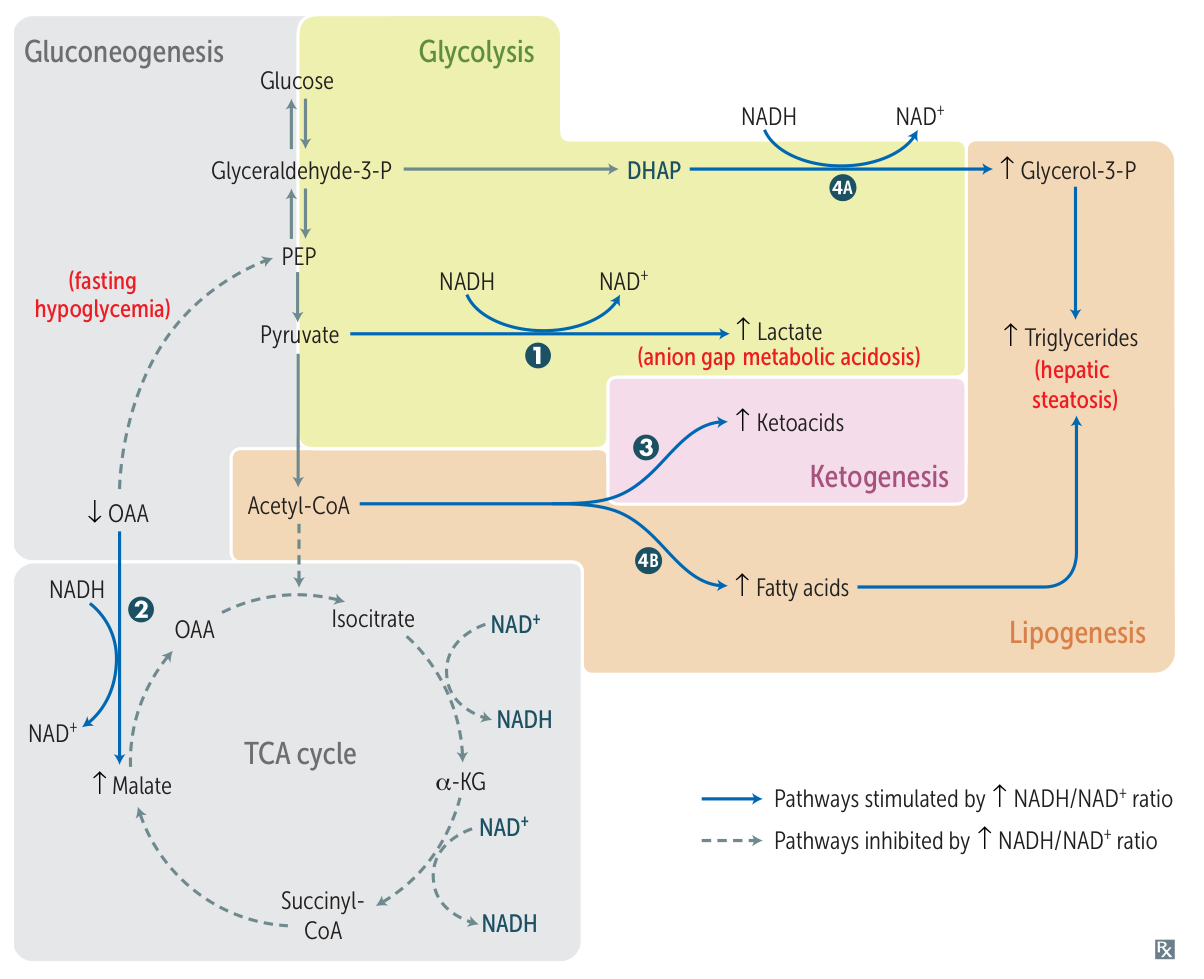
Ethanol metabolism increase NADH/NAD+, which further downregulate gluconeogenesis and TCA cycle, and increase lactate.
Mnemonic
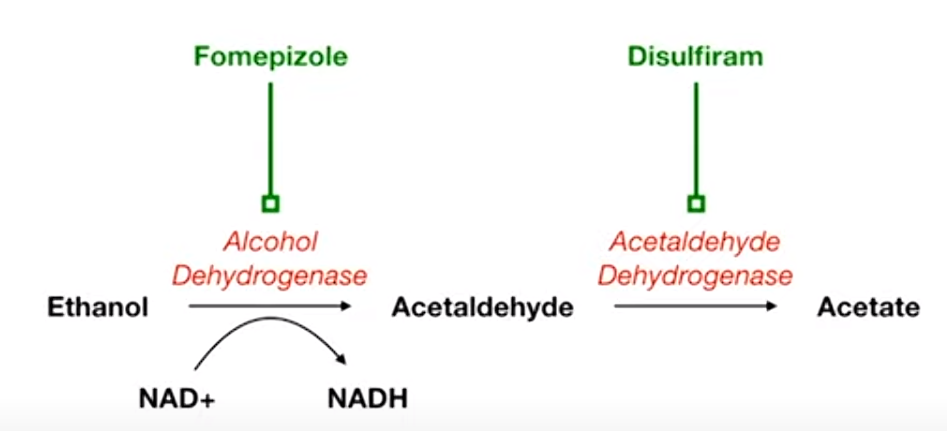
- Fomepizole: think it as Foam-epizole, which can turn the beer to a lot of foam and less liquid (dehydro).
- Used for alcohol intoxication
- Disulfiram: diSUFFERam. It can increase acetaldehyde and make people sick and dizzy.
- Used in alcohol use disorder, as 2nd line treatment
Treatment
Pharmacotherapy
- Psychosocial: Alcoholics Anonymous (AA) and cognitive-behavioral therapy (CBT) are mainstays.
- Withdrawal Management:
- Benzodiazepines: Gold standard (substitution therapy).
- Chlordiazepoxide or Diazepam: Long-acting; preferred if liver function is decent.
- Lorazepam, Oxazepam, Temazepam (LOT): Preferred in liver disease/elderly (metabolized by glucuronidation, not P450).
- Benzodiazepines: Gold standard (substitution therapy).
- Supportive:
- Thiamine (B1) before Glucose to prevent precipitation of Wernicke Encephalopathy.
- Folate, Multivitamins, Mg (Banana bag).
- Long-term / Relapse Prevention:
- Naltrexone: Mu-opioid antagonist. ↓ craving/reward. 1st line. Avoid in acute hepatitis/liver failure.
- Acamprosate: NMDA modulator. ↓ craving. Preferred in liver disease. Contraindicated in renal failure.
- Disulfiram: Aldehyde dehydrogenase inhibitor. Causes accumulation of acetaldehyde (flushing, nausea, hypotension) if alcohol consumed. Conditioned avoidance.
Complications
- CNS Complications
- Wernicke Encephalopathy: Acute, reversible condition. Classic triad: Confusion, Ataxia, Nystagmus/Ophthalmoplegia.
- Pathophysiology: Thiamine (Vitamin B1) deficiency. Affects mammillary bodies.
- Tx: IV thiamine before glucose administration.
- Korsakoff Syndrome: Chronic, often irreversible sequel to Wernicke’s. Characterized by anterograde/retrograde amnesia and confabulation.
- Pathophysiology: Permanent damage to mammillary bodies and thalamic nuclei.
- Cerebellar degeneration: Chronic use leads to degeneration of Purkinje cells in the cerebellar vermis. Cerebellar atrophy manifests as gait ataxia and truncal instability. t
- Central Pontine Myelinolysis (Osmotic Demyelination Syndrome): Demyelination of the pons due to rapid correction of hyponatremia, often seen in malnourished alcoholics. Presents with “locked-in” syndrome.
- Withdrawal:
- Mild (6-24 hrs): Insomnia, tremors, anxiety, diaphoresis.
- Seizures (12-48 hrs): Generalized tonic-clonic.
- Hallucinosis (12-48 hrs): Auditory/visual hallucinations, clear sensorium.
- Delirium Tremens (DTs) (48-96 hrs): Medical emergency. Disorientation, hallucinations, agitation, tachycardia, HTN, fever.
- Wernicke Encephalopathy: Acute, reversible condition. Classic triad: Confusion, Ataxia, Nystagmus/Ophthalmoplegia.
- Gastrointestinal Complications
- Liver Disease:
- Steatosis (Fatty Liver): Reversible. Macrovesicular fat deposits.
- Alcoholic Hepatitis: Jaundice, fever, tender hepatomegaly. Labs: AST > ALT (ratio > 2:1). Histo: Mallory bodies (intracytoplasmic eosinophilic inclusions).
- Cirrhosis: Irreversible fibrosis. Leads to portal HTN (esophageal varices, ascites, caput medusae), hepatic encephalopathy, ↑ risk of hepatocellular carcinoma (HCC).
- Pancreatitis: Acute and chronic pancreatitis are strongly associated with alcohol use.
- Esophagitis/Gastritis: Direct mucosal injury.
- Mallory-Weiss Syndrome: Partial-thickness tear at gastroesophageal junction due to forceful retching. Presents with hematemesis.
- Malabsorption: Leads to various vitamin deficiencies (thiamine, folate, B12, vitamin K).
- Liver Disease:
- Cardiovascular Complications
- Dilated Cardiomyopathy: Alcohol is a direct cardiotoxin. Presents with signs of systolic heart failure (dyspnea, orthopnea, edema).
- Hypertension (HTN): Common dose-dependent effect.
- Atrial Fibrillation: “Holiday Heart Syndrome” - acute arrhythmia after binge drinking.
- Hematologic Complications
- Macrocytic Anemia: Most commonly due to folate deficiency. Can also be from B12 deficiency or direct bone marrow suppression.
- Thrombocytopenia: Due to ↓ production (marrow suppression) and ↑ destruction (splenomegaly from cirrhosis).
- Metabolic/Endocrine Complications
- Alcoholic Ketoacidosis: Seen in chronic alcoholics after a binge, often with poor oral intake. Presents with high anion gap metabolic acidosis.
- Hypoglycemia: Alcohol metabolism depletes NAD+, shunting substrates away from gluconeogenesis.
- Hypertriglyceridemia: Alcohol increases VLDL synthesis.
- Hypogonadism: In males, leads to testicular atrophy, decreased libido, gynecomastia.
- Neoplasia
- Increased risk of cancers of the:
- Oropharynx & Esophagus (squamous cell carcinoma)
- Liver (hepatocellular carcinoma)
- Pancreas
- Colon & Rectum
- Breast (in females)
- Increased risk of cancers of the:
- Fetal Alcohol Syndrome (FAS)
- Leading cause of intellectual disability in the U.S.
- Features: Smooth philtrum, thin vermilion border, small palpebral fissures, microcephaly, intellectual disability, heart defects (VSD).
Alcohol intoxication
Pathophysiology
- Mechanism
- GABA-A receptor agonist → Chronic use causes downregulation of GABA-A receptors → decreased GABA-A activity when cessation
- NMDA glutamate antagonist → chronic use causes upregulation of NMDA receptors → increased glutamate NMDA activity when cessation
- Absorption: The majority of alcohol consumed is absorbed by the proximal small intestine; only a small amount of alcohol is absorbed by the oral, esophageal, and/or gastric mucosa.
Clinical features
Altered consciousness, cognition, perception, judgment, affect, and/or behavior.
Diagnostics
- Routine laboratory studies that support the diagnosis include:
- Elevated osmolar gap
- The difference between the measured serum osmolarity and the calculated serum osmolarity. Normally, the calculated serum osmolarity is primarily determined by circulating levels of sodium salts (chloride and bicarbonate), glucose, and urea. An increased serum osmolar gap occurs when other solutes (e.g., ethanol, methanol, propylene glycol) are present in high enough concentrations to increase the measured osmolarity by more than 10 mOsmol/L.
- Anion gap metabolic acidosis (e.g., ↓ pH, ↓ HCO3-)
- Elevated osmolar gap
Treatment
Alcohol dehydrogenase inhibitors
- Goal: to prevent the conversion of methanol or ethylene glycol to toxic metabolites (e.g., formic acid, glycolic acid, oxalic acid) by competitively inhibiting alcohol dehydrogenase
- Options
- Fomepizole (first-line): a competitive alcohol dehydrogenase inhibitor; safer and easier to administer than ethanol
- Ethanol (second-line)