The nephron
Tip
- Iso-osmotic reabsorption: The proximal tubule reabsorbs sodium and water in equal proportions. This means that while a large amount of sodium is reabsorbed, the concentration in the tubular fluid remains relatively constant.
- Active and passive transport: Sodium reabsorption is driven by active transport mechanisms, primarily the Na+/K+ ATPase pump. This creates an electrochemical gradient that drives the reabsorption of other solutes, including water. Potassium is reabsorbed both passively and actively.
- Regulation: Hormonal mechanisms, such as aldosterone, can fine-tune sodium reabsorption in later parts of the nephron (distal tubule and collecting duct), but in the proximal tubule, the focus is on bulk reabsorption.
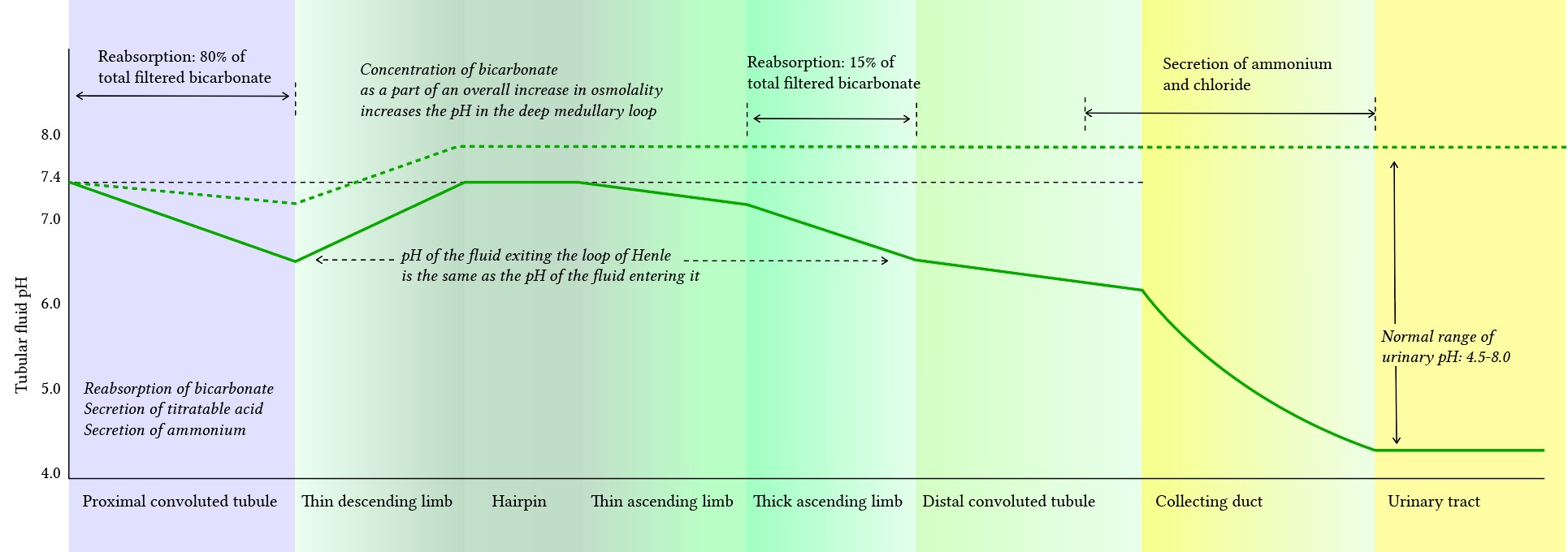
- Renal pH: lowest at collecting duct
Proximal convoluted tubule (PCT)
Tip
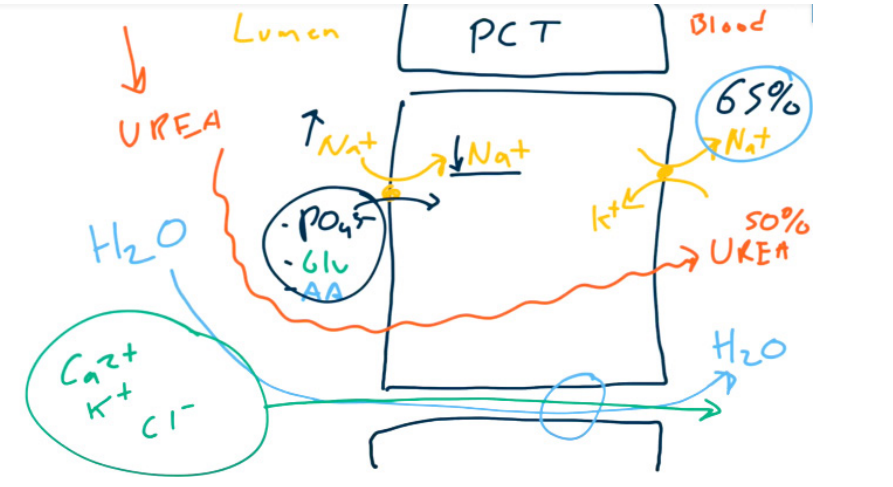
Workhorse: PCT reabsorbs 65-80% of Na, HO (isotonic), HCO, Cl. i.e. most of reabsorption t
- Bicarbonate
- Don’t change H+, only absorb HCO3-
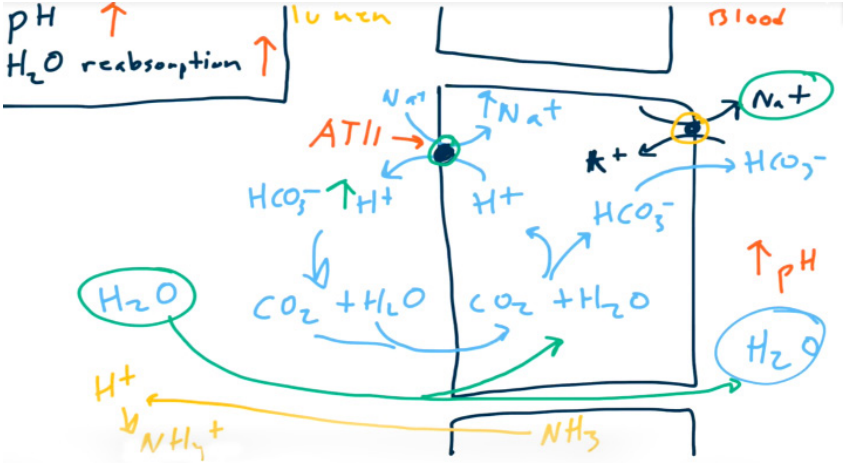
- Absorb Na+ to absorb H2O. 65% of sodium and water are absorbed in PCT.
- Angiotensin II increases Na+, HCO3-, and H2O reabsorption via Na+/H+ exchanger stimulation (allows for contraction alkalosis).
- Don’t change H+, only absorb HCO3-
- Parathormone (PTH) decreases PO43- reabsorption via Na+-PO43- cotransporter inhibition.
- Brush border resorption of most of the ultrafiltrate
- Glucose (via SGLT2)
- Amino acids
- Uric acid
- Na+, Cl-, K+, HCO3-, PO43-, and H2O (All together with Na+ !!!)
Thick ascending loop of Henle
- ADH stimulates the activity of Na+-K+-2Cl- cotransporter. Increased NKCC2 activity aids in water reabsorption in the collecting duct through aquaporin 2 channels by creating a hypo-osmotic filtrate.
Distal convoluted tubule (DCT)
- Magnesium and Calcium are reabsorbed paracellularly.
- Ca2+ is reabsorbed using Ca2+ channels on the luminal surface and Na+/Ca2+ antiporters (exchangers) on the basolateral surface.
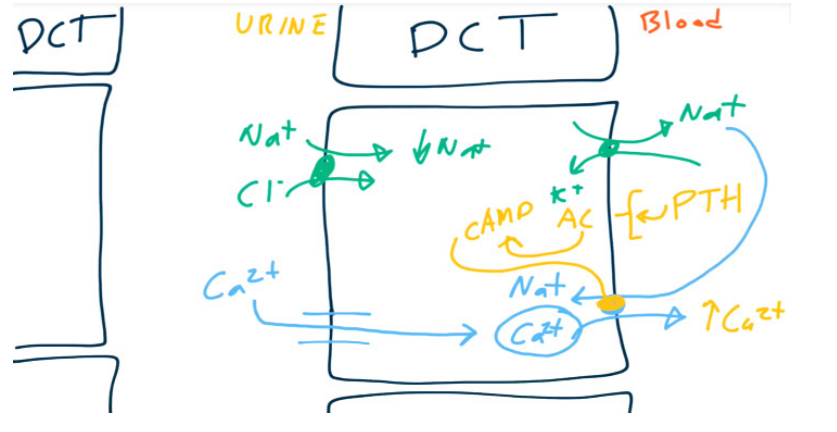
- PTH up-regulates the Ca2+/Na+ antiporter resulting in increased reabsorption of Ca2+.
Collecting duct
- Principal cells
- When acted upon by ADH, principal cells will increase the number of aquaporins (water channels) on the luminal membrane → increased H2O reabsorption.
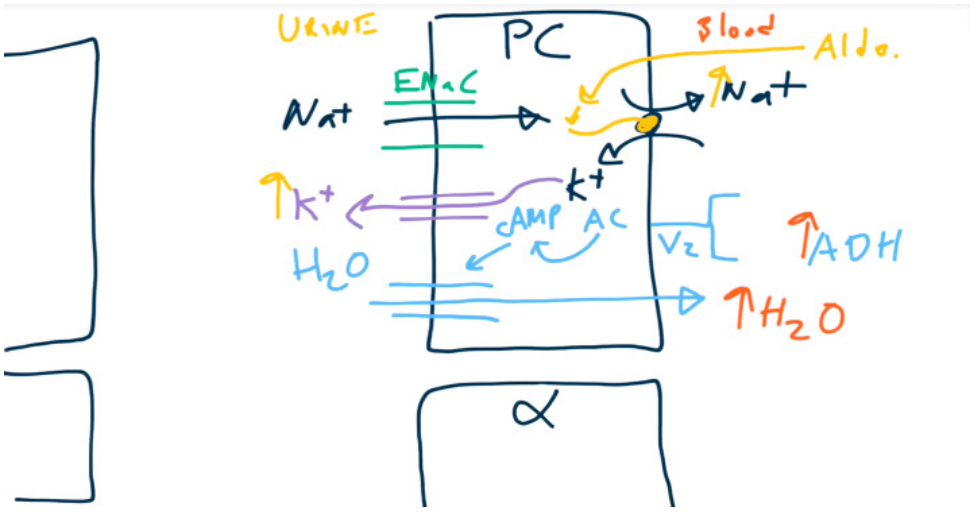
- When acted upon by aldosterone, principal cells increase activity of Na+/K+ exchangers → increased Na+ reabsorption and increased K+ secretion.
- Water follows sodium through the water channels. So aldosterone won’t affect the urine osmolality.
- When acted upon by ADH, principal cells will increase the number of aquaporins (water channels) on the luminal membrane → increased H2O reabsorption.
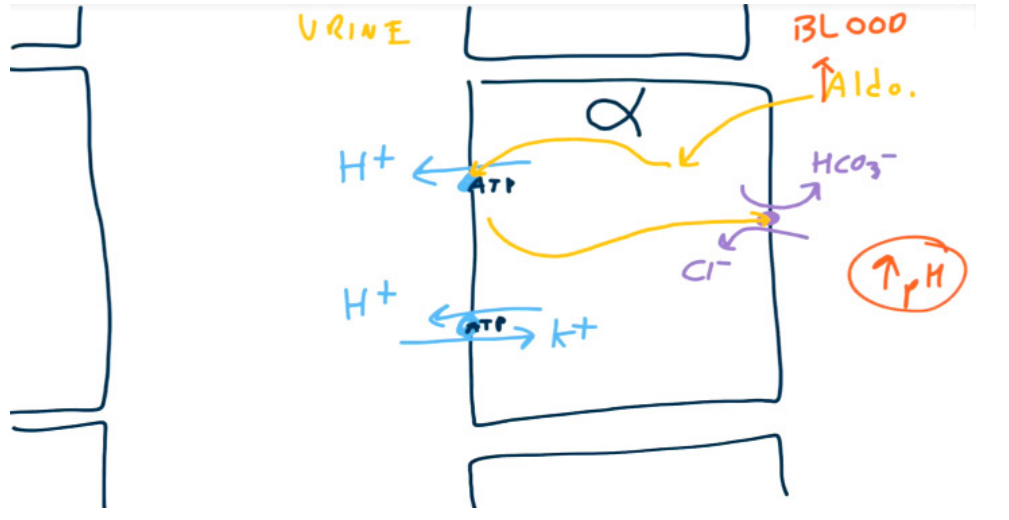
- Alpha-intercalated cells
- Have a K+/H+ exchanger on the luminal surface to secrete H+ and reabsorb K+
- Have a H+-ATPase which actively secretes H+. On the basolateral surface, the cells have a HCO3−/Cl- exchanger to reabsorb HCO3−.
- Aldosterone upregulates H+ secretion via H+-ATPase. Increased H+ secretion will lead to increased HCO3- reabsorption.
- ADH also stimulates reabsorption of urea in collecting ducts to maximize corticopapillary osmotic gradient. So increased BUN-creatinine ratio in hypovolemia.
- Urea passively diffuses from the interstitium into the loop of Henle, increasing the luminal concentration of urea.
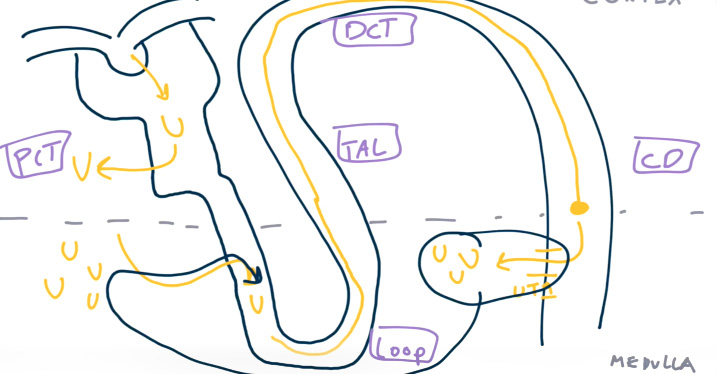
- Urea passively diffuses from the interstitium into the loop of Henle, increasing the luminal concentration of urea.
Tip
K+ and H+ are in a competitive displacement relationship with the Na+ found in renal tubular urine. This means that the ion (either K+ or H+) with the higher concentration will exchange with the Na+.
Renal blood flow
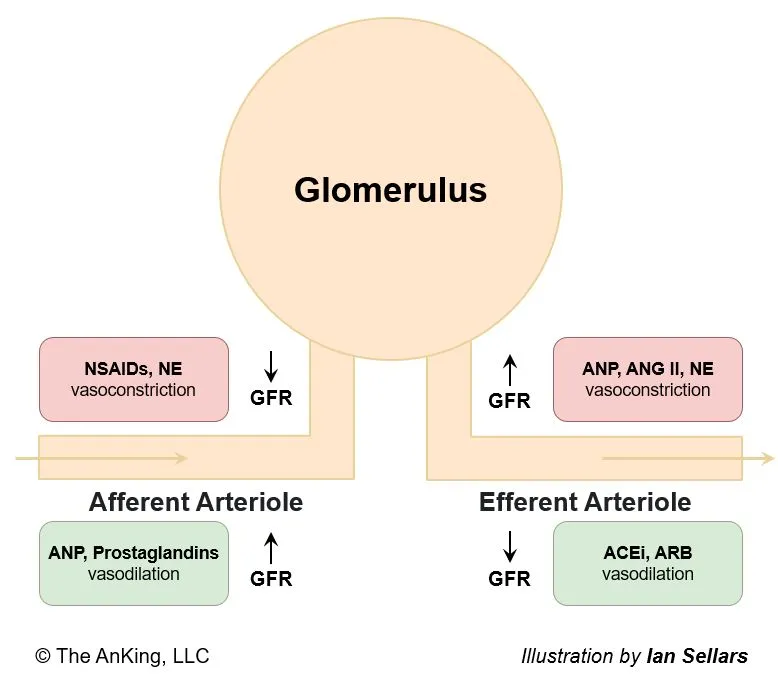
Mnemonic
Physiological substances all increase GFR, while artificial medications all reduce GFR.
Tubuloglomerular feedback
- Description: feedback system between the tubules and glomeruli that adjusts the GFR according to the resorption capacity of the tubules
- Mechanism: macula densa (of the juxtaglomerular apparatus) senses alterations in the NaCl concentration in the DCT
- Hypotonic urine (↓ intraluminal Cl- concentration) → vasodilation of afferent arterioles → ↑ GFR → ↑ Cl- intraluminal concentration → ↑ RBF
- Hypertonic urine (↑ intraluminal Cl- concentration) → adenosine secretion → vasoconstriction of afferent arterioles → ↓ capillary pressure → ↓ GFR → ↓ intraluminal Cl- concentration → ↓ RBF
Juxtaglomerular complex
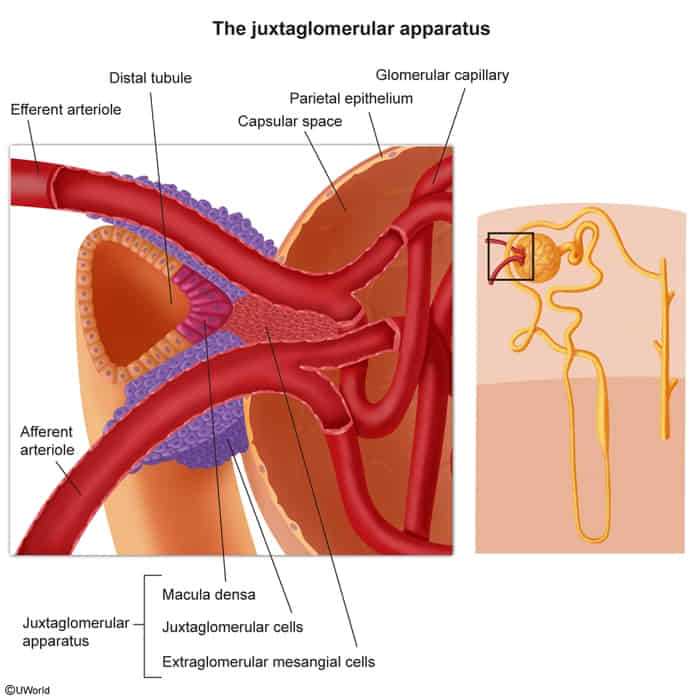
- Structure located where the distal convoluted tubule (DCT) meets the afferent arteriole.
- Macula Densa: NaCl sensors in the DCT wall.
- Juxtaglomerular (JG) Cells: Modified smooth muscle cells in the afferent arteriole that secrete renin. t
- Extraglomerular Mesangial Cells (Lacis Cells): Mesangial cells that signal between the other two cell types.
Myogenic autoregulation (Bayliss effect)
- Definition
- Intrinsic vascular smooth muscle contraction in response to stretch (increased blood pressure).
- Goal: Maintain constant blood flow (autoregulation) despite changes in perfusion pressure.
- Mechanism
- Vessel distension → Opens stretch-activated cation channels → Depolarization.
- Opens voltage-gated L-type Ca2+ channels → ↑ Intracellular Ca2+.
- Result: Vasoconstriction.
- Key Locations
- Kidney: Afferent arteriole (protects glomerulus, stabilizes GFR).
- Brain: Cerebral arteries (maintains cerebral perfusion pressure).
- Clinical Relevance
- Hypertension: Chronic HTN shifts autoregulation curve to the right (requires higher BP to maintain flow).
- Dihydropyridines (e.g., Amlodipine): Block the L-type Ca2+ channels involved in this reflex.
Measurement of renal function
Filtration fraction
- Description: the fraction of the renal plasma flow (RPF) that is filtered from the capillaries into the Bowman space
- Mechanism
- FF = GFR/RPF (i.e., FF = CInulin/CPAH) t
- GFR can be calculated using the inulin or creatinine clearance, as these substances are freely filtered at the glomerulus and have relatively insignificant tubular reabsorption or secretion. RPF can be determined using the para-aminohippuric acid (PAH) clearance as almost all the PAH entering the kidneys is excreted in the urine (mostly via tubular secretion).
- CInulin = inulin clearance
- CPAH = PAH clearance
- Normal: 20%
- Regulated via:
- Prostaglandins → dilation of afferent arterioles → ↑ GFR and ↑ RPF (FF unchanged)
- Angiotensin II → constriction of efferent arterioles → ↑ GFR and ↓ RPF → ↑ FF
- FF = GFR/RPF (i.e., FF = CInulin/CPAH) t
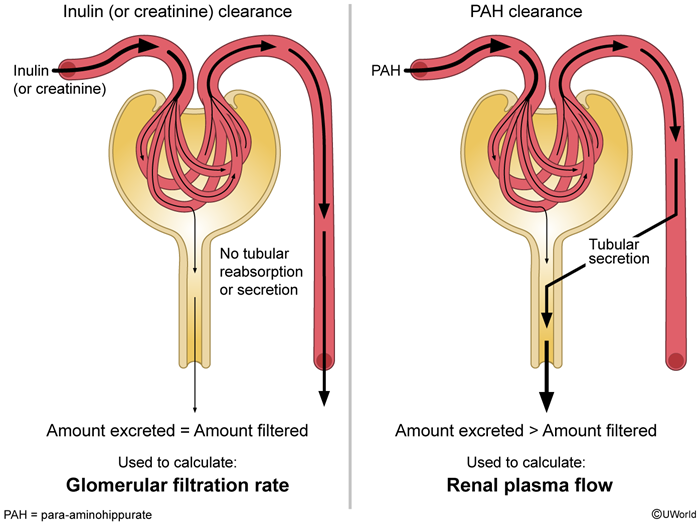
Renal clearance
- Renal Clearance (Cx)
- The volume of plasma from which a substance is completely cleared per unit time.
- Formula: Cx = (Ux * V) / Px
- Ux = Urine concentration of substance X
- V = Urine flow rate
- Px = Plasma concentration of substance X
- If Cx < GFR → Net tubular reabsorption of X.
- If Cx > GFR → Net tubular secretion of X.
- If Cx = GFR → No net reabsorption or secretion.
| Feature | Filtered Load | Excretion Rate | Clearance |
|---|---|---|---|
| What it measures | Amount of substance entering the nephron | Amount of substance leaving the body | Volume of plasma “cleaned” of the substance |
| Formula | GFR × Pₓ | Uₓ × V | (Uₓ × V) / Pₓ |
| Units | mg/min | mg/min | mL/min |
| Analogy | The total amount of trash thrown into a factory’s processing line. | The amount of trash that actually comes out the other end into the dumpster. | How many garbage trucks’ worth of trash the factory processed (normalized to concentration). |
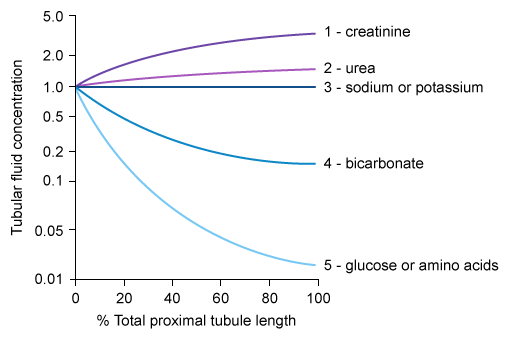
Relative solute concentrations along proximal convoluted tubules
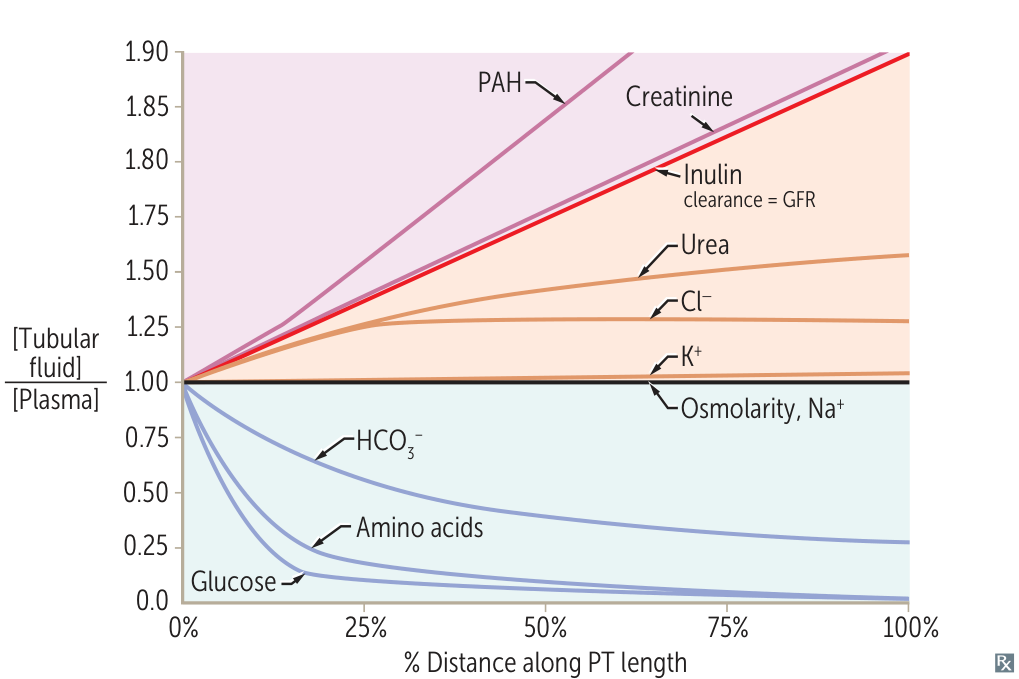
- TF/P < 1 (Reabsorbed faster than water)
- Glucose & Amino Acids: Drop rapidly to ~0 (Avid Na+-cotransport).
- HCO3-: Drops to ~0.4 (Reclaimed to maintain pH).
- TF/P = 1 (Reabsorbed at same rate as water)
- Na+ & Osmolarity: Flat line (Isotonic reabsorption).
- K+: Roughly flat. t
- TF/P > 1 (Reabsorbed slower than water or Secreted)
- Cl-: Rises slightly (slower reabsorption).
- Urea: Rises moderate (poor reabsorption).
- Inulin: Rises to ~3.0 (Filtered only; marker of water reabsorption).
- Creatinine & PAH: Rise steeply (PAH is highest due to active secretion).
Kidney embryology
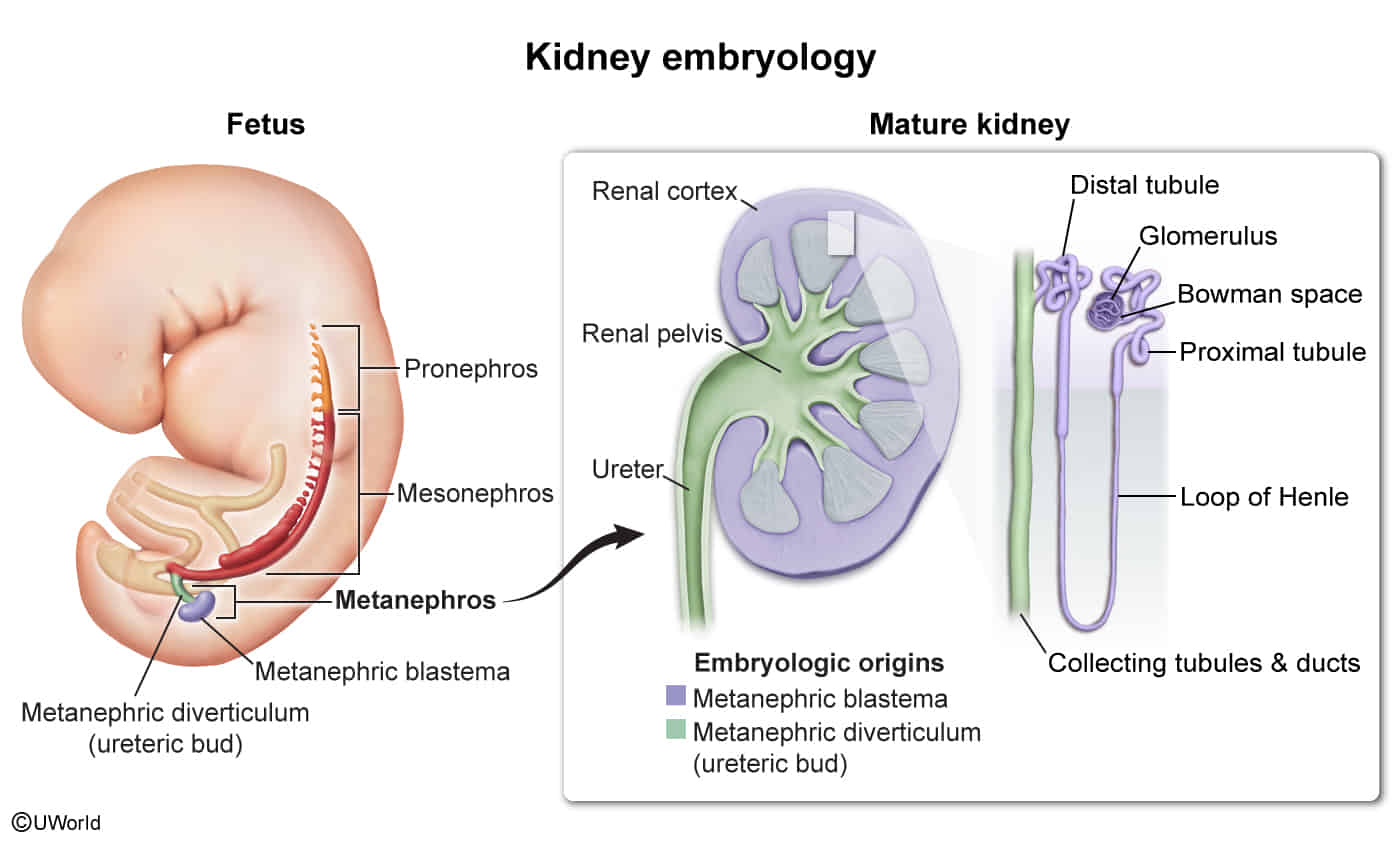
- Developmental Sequence t
- Occurs in 3 successive stages from intermediate mesoderm, cranial to caudal:
- Pronephros: Week 4; then degenerates completely.
- Mesonephros: Functions as interim kidney for 1st trimester; later contributes to male genital system (Wolffian duct → ductus deferens, epididymis).
- Metanephros: Appears at week 5; forms the permanent kidney. Nephrogenesis continues through 32-36 weeks of gestation.
- Occurs in 3 successive stages from intermediate mesoderm, cranial to caudal:
- Metanephros (Permanent Kidney) Development
- Develops via reciprocal induction between two key structures:
- 1. Ureteric Bud (Metanephric Diverticulum)
- Outgrowth from the caudal end of the mesonephric (Wolffian) duct.
- Gives rise to the collecting system:
- Ureter
- Pelvis (Renal Pelvis)
- Calyces (Major and Minor)
- Collecting Ducts
- Ureteric bud is induced to grow and branch by the metanephric mesenchyme.
- Aberrant interaction can lead to multicystic dysplastic kidney.
- 2. Metanephric Mesenchyme (Metanephric Blastema)
- Mesoderm that interacts with the ureteric bud.
- Differentiates into the kidney’s filtration system (the nephron):
- Glomerulus
- Bowman’s space/capsule
- Proximal convoluted tubule (PCT)
- Loop of Henle
- Distal convoluted tubule (DCT)
- Differentiation is induced by signaling from the ureteric bud.
- Ascent of the Kidneys
- Kidneys originate in the pelvis and ascend to their final position in the upper abdomen.
- During ascent, they establish a new, more superior blood supply from the aorta. The original inferior vessels degenerate.
Kidney anatomy
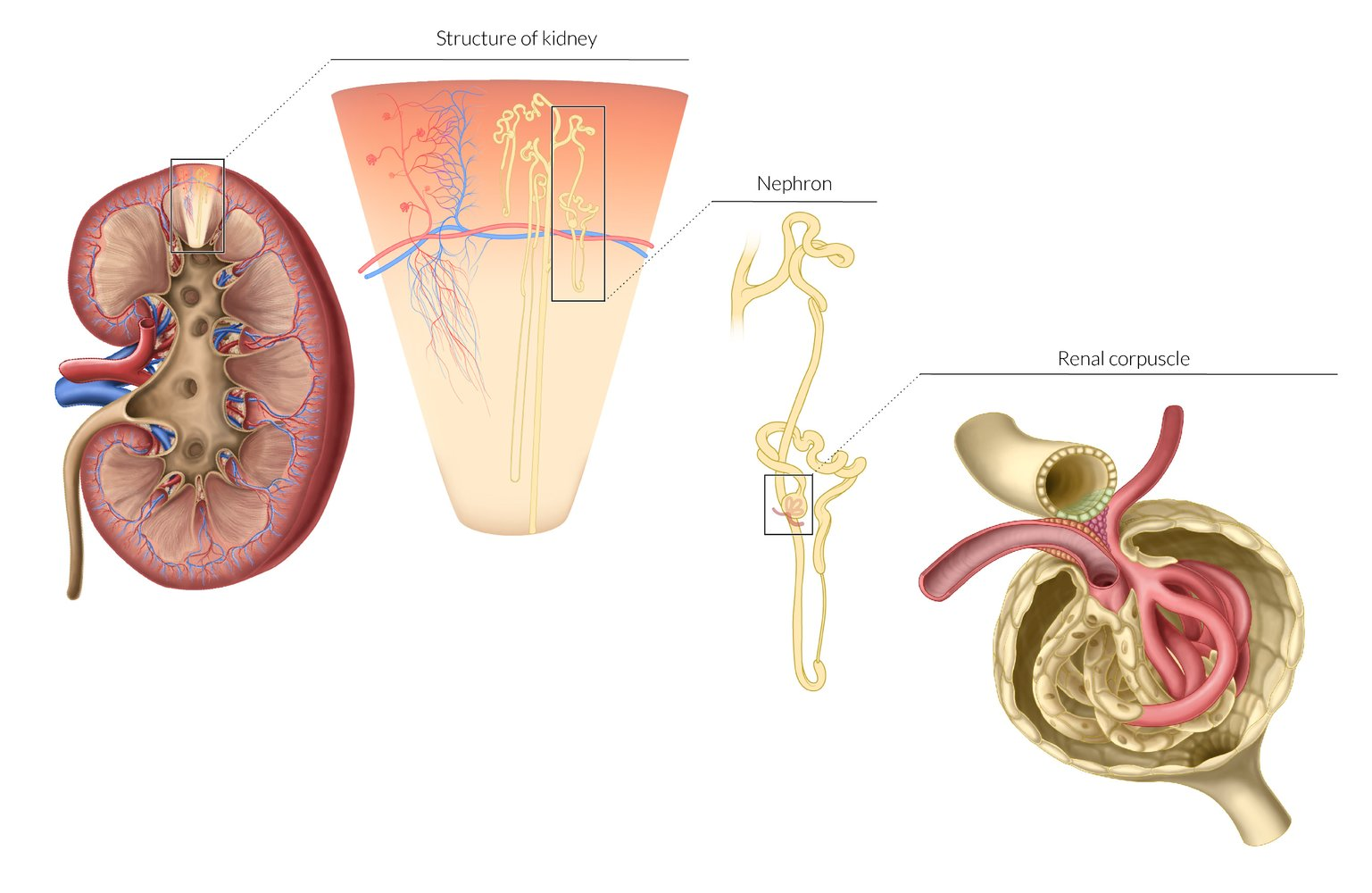
- Cortex: Outer region containing the glomeruli and convoluted tubules. Its tissue extends between the pyramids as renal columns.
- Medulla: Inner region of the kidney, consisting of renal pyramids. It contains the structures responsible for maintaining salt and water balance.
- Renal Pyramids: Cone-shaped tissues of the medulla. Their striped appearance is due to the parallel arrangement of nephron loops and collecting ducts. The base of each pyramid faces the cortex, and the apex (papilla) points toward the renal pelvis.
- Papilla: The apex of each pyramid, which projects into a minor calyx, allowing urine to pass into it.
- Calyces: Minor calyces collect urine from the papillae. Several minor calyces merge to form a major calyx.
- Renal Pelvis: Funnel-shaped structure formed by the union of major calyces, which narrows to become the ureter at the ureteropelvic junction (UPJ).
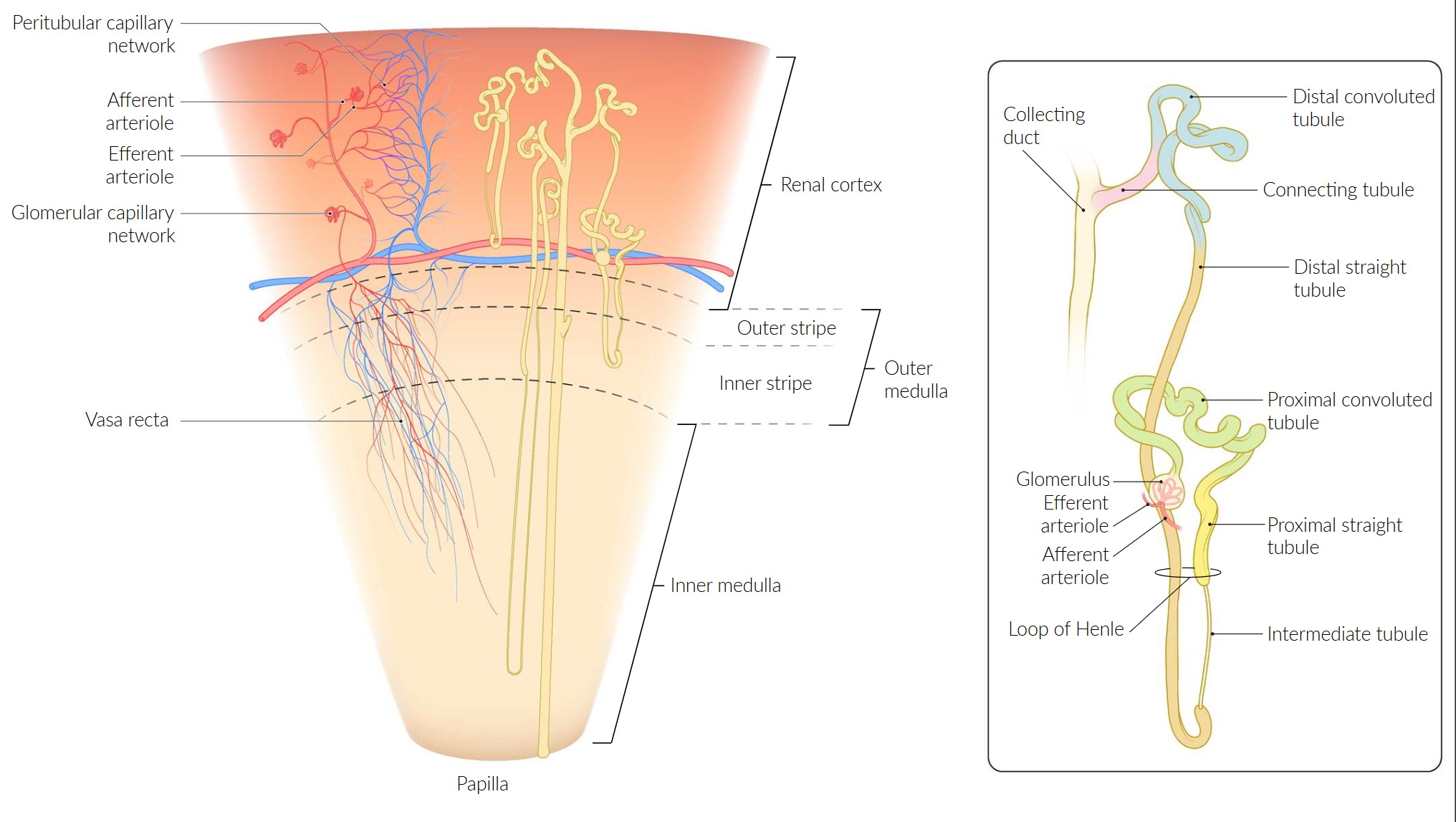
- Renal cortex
- The outermost layer of the renal parenchyma (∼ 10 mm thick)
- Surrounds the renal medulla and extends inwards (as renal columns) dividing the medulla into renal pyramids
- Contains the glomeruli, proximal convoluted tubules, distal convoluted tubules, and cortical collecting ducts
- Renal medulla
- Consists of several renal medullary pyramids separated by the renal columns
- The base of each pyramid faces the outer cortex, the apex faces the renal sinus and forms a renal papilla, which drains into a minor calyx.
- Contains the loops of Henle and collecting ducts, which merge to form the papillary ducts at the renal papillae
- Blood flow in the renal medulla is relatively low compared to that in the renal cortex.
- Facilitates the development of an osmolality gradient that allows for effective urine concentration
- Causes medullary vulnerability to hypoxia when renal blood flow is decreased (renal ischemia)
- Renal sinus
- The inner portion of the kidney containing the renal calyces and renal pelvis
- The minor calyces draining from each renal papilla merge to form major calyces.
- The major calyces merge to form the renal pelvis, which represents the proximal portion of the ureter.
- Renal hilum: The medial fissure on each kidney where the renal pelvis, vessels, and nerves enter and exit
Hormone synthesis
- Erythropoietin (EPO)
- Source: Peritubular interstitial cells (fibroblast-like cells in the renal cortex).
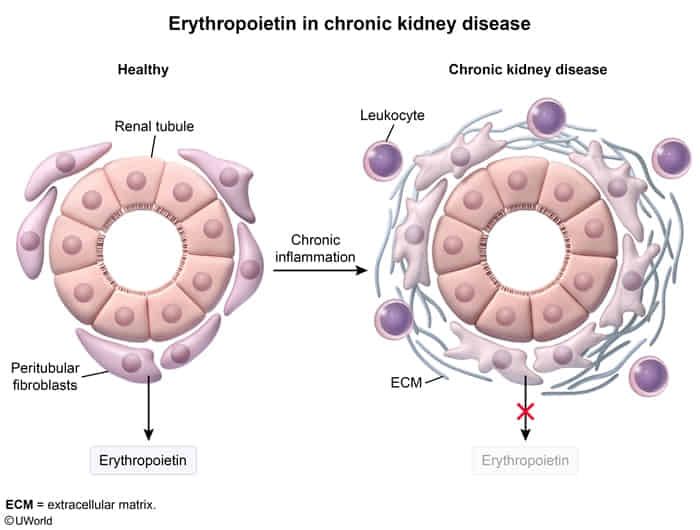
- Trigger: Hypoxia.
- Clinical: ↓ in CKD (causes anemia); ↑ in RCC (polycythemia).
- Source: Peritubular interstitial cells (fibroblast-like cells in the renal cortex).
- 1,25-Vitamin D (Calcitriol)
- Source: Proximal Convoluted Tubule (PCT) cells (specifically within mitochondria).
- Trigger: ↑ PTH (major), ↓ Ca2+, ↓ PO₄³⁻.
- Clinical: Deficiency in CKD → Secondary Hyperparathyroidism.
- Renin
- Source: Juxtaglomerular (JG) cells (modified smooth muscle cells of the afferent arteriole).
- Trigger: ↓ Renal perfusion, ↓ NaCl (Macula Densa), ↑ Sympathetic (β1).
- Function: RAAS activation (↑ BP).
- Prostaglandins (PGE2)
- Source: Renal Medullary Interstitial Cells and Collecting Duct cells.
- Function: Dilates Afferent arteriole (protects RBF).
- Clinical: NSAIDs inhibit PGs → afferent constriction → Acute Kidney Injury.
- Dopamine
- Source: Proximal Convoluted Tubule (PCT) cells (synthesized from circulating L-DOPA).
- Function: Natriuresis (↑ Na⁺ excretion) and vasodilation (at low doses).