Pattern Recognition Receptors (PRRs)
Innate immune receptors that recognize conserved Pathogen-Associated Molecular Patterns (PAMPs) and Damage-Associated Molecular Patterns (DAMPs), triggering inflammatory responses via transcription factors NF-κB and IRFs.
- Toll-Like Receptors (TLRs)
- Function: Recognize extracellular or endosomal pathogens. Signal mainly via MyD88 to activate NF-κB.
- Key Examples:
- TLR4: On cell surface; recognizes LPS from Gram-negative bacteria.
- TLR2: On cell surface; recognizes peptidoglycan/lipoteichoic acid from Gram-positive bacteria.
- TLR3, 7, 8: In endosomes; recognize viral nucleic acids (dsRNA, ssRNA).
- NOD-Like Receptors (NLRs)
- Function: Cytosolic sensors of intracellular pathogens and cell damage.
- Key Examples:
- NOD2: Recognizes bacterial peptidoglycan. Mutations are strongly associated with Crohn’s disease.
- NLRP3 Inflammasome: A cytosolic complex activated by diverse DAMPs (e.g., uric acid crystals). Its activation leads to caspase-1 activation, which cleaves pro-IL-1β into active IL-1β. This pathway is central to the inflammation seen in gout.
- RIG-I-Like Receptors (RLRs)
- Function: Cytosolic sensors specializing in viral RNA. Their activation is crucial for producing Type I Interferons (IFN-α/β).
- Key Example:
- RIG-I: Recognizes specific RNA structures common in viral genomes (e.g., 5’-triphosphate RNA).
- High-Yield Associations
- TLR4 Deficiency: Increased susceptibility to Gram-negative sepsis.
- NOD2 Mutation: Increased risk for Crohn’s disease, particularly ileal disease.
- NLRP3/Inflammasome: Gout (activated by uric acid crystals), autoinflammatory syndromes.
NF-κB pathway
- Function
- Master pro-inflammatory transcription factor.
- Drives expression of genes for inflammation (cytokines), immunity, and cell survival (anti-apoptosis).
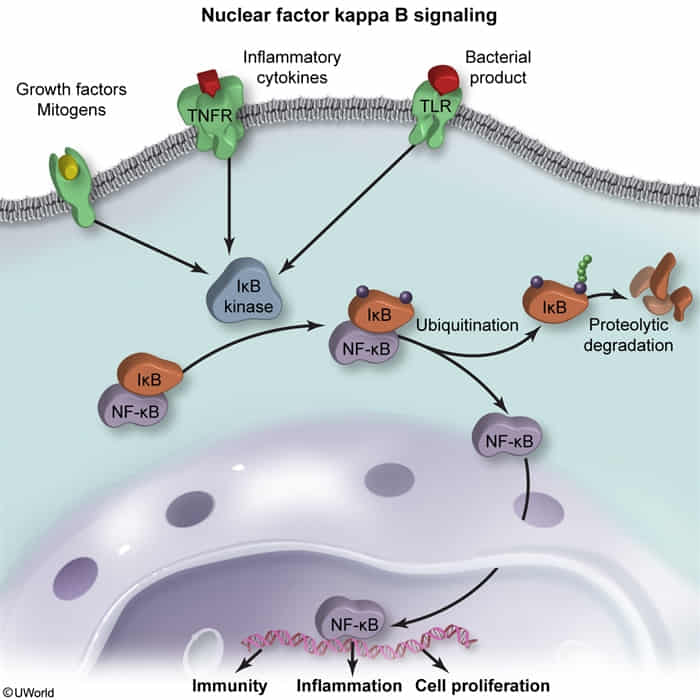
- Canonical Pathway (Most Common)
- Resting Cell: NF-κB is held inactive in the cytoplasm by its inhibitor, IκB.
- Activation State:
- Triggers (TNF-α, IL-1, LPS) activate IKK (IκB Kinase).
- IKK phosphorylates IκB.
- Phosphorylated IκB is ubiquitinated and degraded by the proteasome.
- Freed NF-κB translocates to the nucleus to initiate gene transcription.
- Key Gene Products
- Pro-inflammatory cytokines: TNF-α, IL-1, IL-6
- Chemokines & Adhesion Molecules
- Anti-apoptotic proteins (e.g., B-cell lymphoma 2 [Bcl-2] family)
- Inhibition & Clinical Relevance
- Negative Feedback: NF-κB induces transcription of its own inhibitor, IκBα, to terminate the signal.
- Glucocorticoids: Inhibit NF-κB by increasing IκBα production.
- Proteasome Inhibitors (e.g., Bortezomib): Block IκB degradation, trapping NF-κB in the cytoplasm.
- Pathology: Dysregulation leads to chronic inflammation (RA, IBD), cancer, and septic shock (due to massive cytokine release from LPS stimulation).