- Overview
- The secretory pathway directs newly synthesized proteins destined for secretion, insertion into the cell membrane, or delivery to lysosomes.
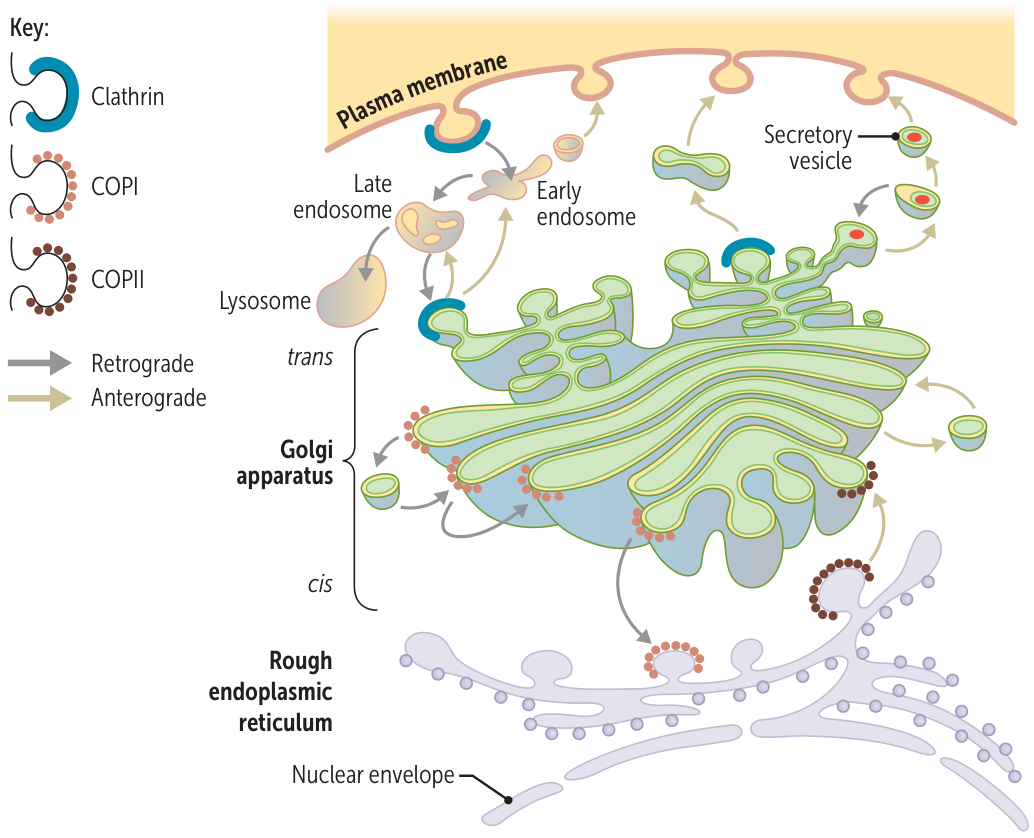
- The canonical route is: Ribosome → Rough ER → Golgi Apparatus → Secretory Vesicles → Plasma Membrane.
- Rough Endoplasmic Reticulum (RER)
- Signal Peptide: Synthesis begins on a cytosolic ribosome. An N-terminal amino acid “signal sequence” directs the ribosome to dock with the RER. This sequence is recognized by a Signal Recognition Particle (SRP).
- Translocation & Folding: The growing polypeptide chain is threaded into the RER lumen. Within the RER, the protein folds into its 3D structure, assisted by chaperone proteins (e.g., BiP).
- Modifications:
- N-linked glycosylation: Addition of an oligosaccharide to the nitrogen atom of asparagine residues. This is a crucial step for proper folding and further sorting.
- Disulfide bond formation.
- Transport from RER to Golgi
- Anterograde Transport: Vesicles coated with COPII proteins bud off from the RER and transport correctly folded proteins to the cis-Golgi network.
- Retrograde Transport: Vesicles coated with COPI proteins return escaped RER-resident proteins (and vesicle components) from the Golgi back to the RER.
- “Two (COPII) steps forward (anterograde); one (COPI) step back (retrograde).”
- Golgi Apparatus
- Function: Acts as the central processing and sorting station for proteins. It modifies, packages, and tags proteins for their final destinations.
- Structure & Flow: Proteins move sequentially through the Golgi stacks: cis → medial → trans.
- Modifications:
- Modification of N-linked oligosaccharides added in the RER.
- O-linked glycosylation: Addition of sugars to the oxygen atom of serine or threonine residues.
- Mannose-6-Phosphate (M6P) Tagging: In the cis-Golgi, proteins destined for lysosomes are tagged with M6P. This tag acts like a specific “zip code.”
- Post-Golgi Trafficking
- Sorting: In the trans-Golgi network, proteins are sorted into different vesicles based on their destination.
- Lysosomal Targeting: M6P receptors in the trans-Golgi bind to the M6P-tagged proteins, packaging them into clathrin-coated vesicles destined for the lysosome.
- Secretion:
- Constitutive Secretion: The “default” pathway. Vesicles are continuously sent to the plasma membrane and fuse without a specific signal. This pathway is used for components of the extracellular matrix and plasma membrane proteins.
- Regulated Secretion: Proteins (e.g., hormones like insulin, neurotransmitters) are stored in secretory granules. These granules only fuse with the plasma membrane and release their contents in response to an external signal (e.g., a hormone or nerve impulse).
- Key Clinical Correlations
- I-Cell Disease (Mucolipidosis II)
- Patho: Autosomal recessive deficiency of N-acetylglucosaminyl-1-phosphotransferase. This enzyme is required for the M6P tagging of lysosomal enzymes in the Golgi.
- Result: Lysosomal enzymes are not sent to the lysosomes. Instead, they are secreted extracellularly via the default constitutive pathway. Substrates that should be degraded accumulate within lysosomes, forming characteristic “Inclusion-cells”.
- Presentation: Appears in infancy with failure to thrive, coarse facial features, skeletal abnormalities (e.g., claw-hand deformity), and severe psychomotor retardation. Lab findings show high levels of lysosomal enzymes in the plasma.
- Cystic Fibrosis (CF)
- Patho: Caused by mutations in the CFTR gene. The most common mutation (ΔF508) leads to misfolding of the CFTR protein in the RER.
- Result: The misfolded protein is recognized by the quality control system and targeted for premature degradation by the proteasome. It never reaches the plasma membrane to function as a chloride channel. This is a classic example of failed protein trafficking leading to disease.
