- Definition: In a multi-subunit protein, the binding of a ligand to one site influences the binding affinity of other sites.
- Positive Cooperativity
- Binding of one ligand increases the affinity for the next ligand.
- Classic Example: Hemoglobin (Hb) and O2.
- Hb is a tetramer (α2β2) that can bind four O2 molecules.
- Binding the first O2 shifts Hb from a low-affinity T (tense) state to a high-affinity R (relaxed) state, making subsequent O2 binding easier.
- This allows Hb to effectively load O2 in the lungs (high pO2) and unload it in tissues (low pO2).
- Graphical Representation
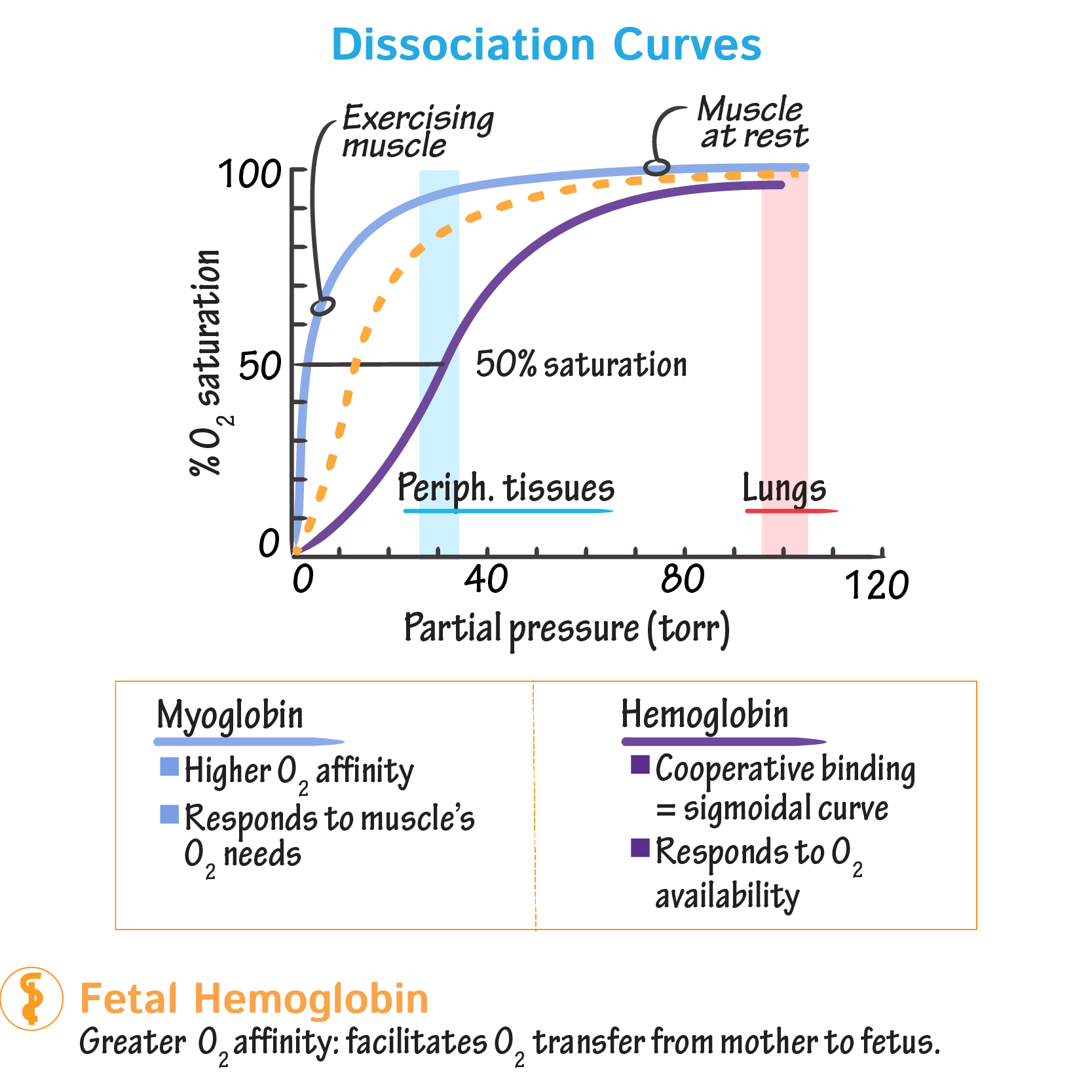
- Sigmoidal Curve: The hallmark of positive cooperativity is an S-shaped binding curve.
- This contrasts with the hyperbolic curve of non-cooperative proteins like Myoglobin.
- Key Regulators of Hb-O2 Affinity
- Factors that decrease O2 affinity (stabilize the T-state and cause a rightward shift of the curve, promoting O2 unloading):
- ↑ 2,3-BPG
- ↑ H+ (↓ pH) - Bohr effect
- ↑ CO2
- ↑ Temperature
- Hill Coefficient (nH)
- A measure of the degree of cooperativity.
- nH > 1: Positive cooperativity (Hemoglobin).
- nH = 1: No cooperativity (Myoglobin).
- nH < 1: Negative cooperativity.
