Receptor binding
- Core Definitions
- Affinity: How strongly a drug binds (↓Kd = ↑Affinity).
- Potency: How much drug is needed for an effect (↓EC50 = ↑Potency). Reflected by a left-shift on a dose-response curve.
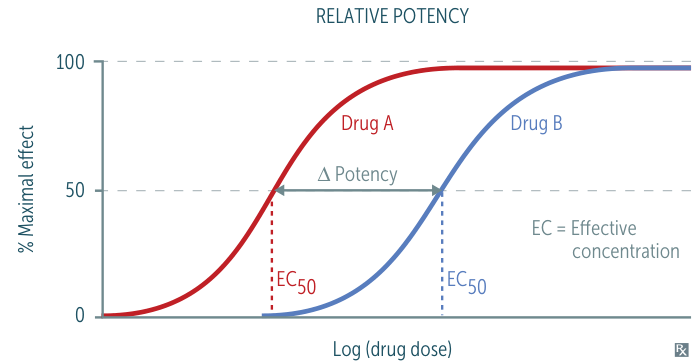
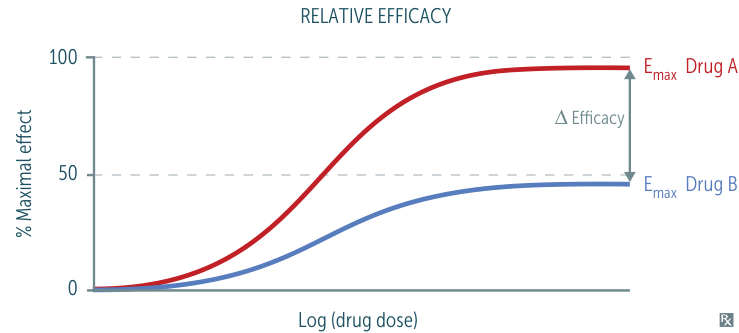
- Efficacy: The maximal effect a drug can produce (↑Emax = ↑Efficacy). Reflected by a higher plateau on a dose-response curve.
- Agonists
- Full Agonist: Produces maximal response. Efficacy = 100%. (e.g., Morphine)
- Partial Agonist: Produces a sub-maximal response. Can act as an antagonist when co-administered with a full agonist. (e.g., Buprenorphine)
- Inverse Agonist: Binds to the receptor and produces the opposite effect of an agonist. Reduces constitutive (baseline) receptor activity. (e.g., some antihistamines on the H1 receptor).
- Antagonists
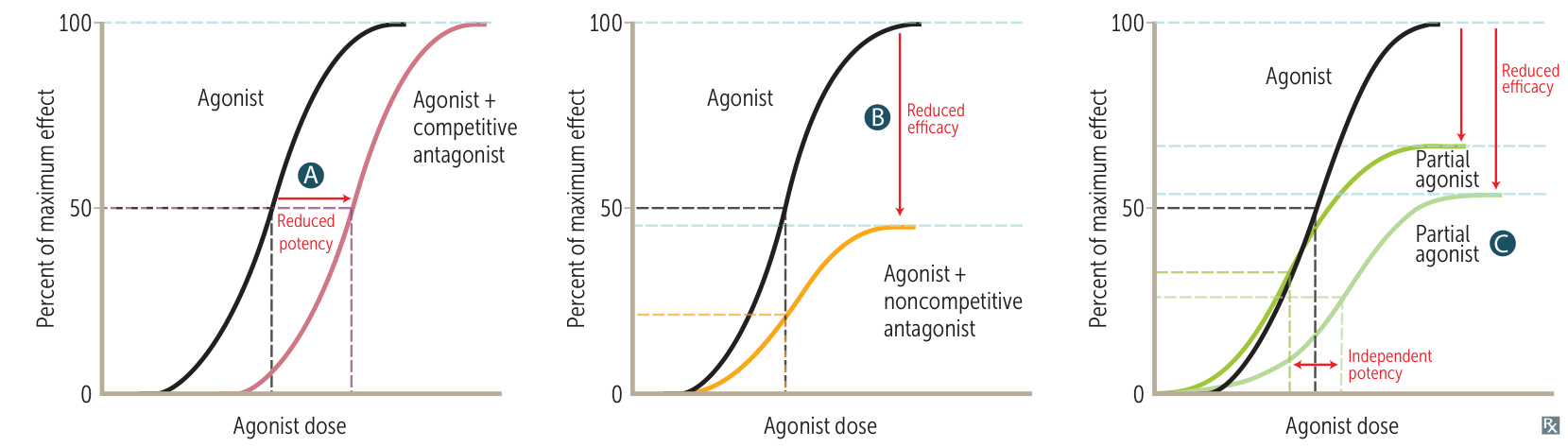
- Competitive Antagonist:
- Binds reversibly to the active site.
- Effect is overcome by ↑ agonist.
- Shifts curve right (↓ Potency). No change in Efficacy (Emax).
- Non-competitive Antagonist:
- Binds irreversibly or to an allosteric site.
- Effect cannot be overcome by ↑ agonist.
- Shifts curve down (↓ Efficacy). No change in Potency (EC50).
- Competitive Antagonist:
| AGONIST WITH | POTENCY | EFFICACY | REMARKS | EXAMPLE |
|---|---|---|---|---|
| (A) Competitive antagonist | ↓ | No change | Can be overcome by ↑ agonist concentration | Diazepam (agonist) + flumazenil (competitive antagonist) on GABAₐ receptor. |
| (B) Noncompetitive antagonist | No change | ↓ | Cannot be overcome by ↑ agonist concentration | Norepinephrine (agonist) + phenoxybenzamine (noncompetitive antagonist) on α-receptors. |
| (C) Partial agonist (alone) | Independent | ↓ | Acts at same site as full agonist | Morphine (full agonist) vs buprenorphine (partial agonist) at opioid μ-receptors. |
| Inverse agonist (alone) | Independent | Independent | Binds to a constitutively active receptor, thereby reducing its activity; has the opposite effect of an agonist | H1 antihistamines (eg, diphenhydramine) |
Tip
- A partial agonist binds to and activates a receptor, but with lower efficacy (intrinsic activity) than a full agonist.
- It can never produce the maximal response, even at full receptor occupancy.
- Dual Action:
- In the absence of a full agonist: It acts as an agonist, producing a submaximal effect.
- In the presence of a full agonist: It acts as a competitive antagonist by competing for receptor binding sites. This reduces the overall effect of the full agonist, bringing the net response down to the level of the partial agonist’s own maximal effect.
Enzyme kinetics
- Key Parameters
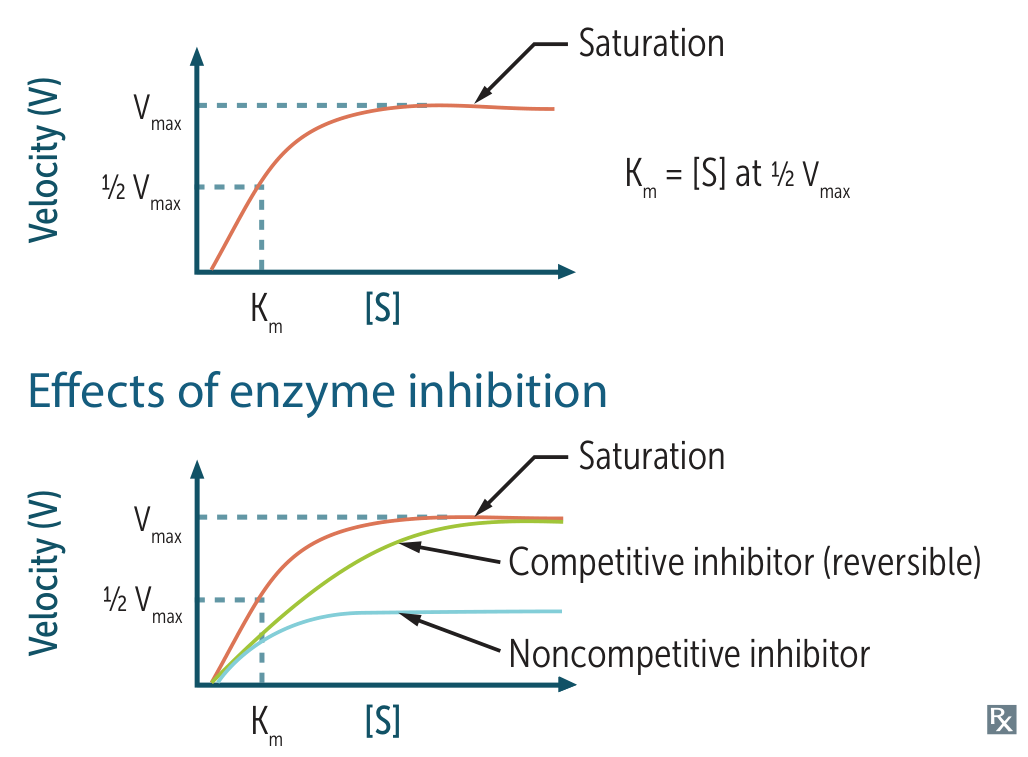
- (Maximum Velocity)
- The rate of reaction when the enzyme is saturated with substrate.
- Directly proportional to enzyme concentration .
- (Michaelis Constant)
- The substrate concentration at which reaction velocity is .
- Inversely related to affinity of the enzyme for its substrate.
- = High Affinity (requires less substrate to reach ).
- = Low Affinity.
- (Maximum Velocity)
- Graphical Representations
- Michaelis-Menten Plot
- Velocity () vs. Substrate Concentration ().
- Typically a hyperbolic curve.
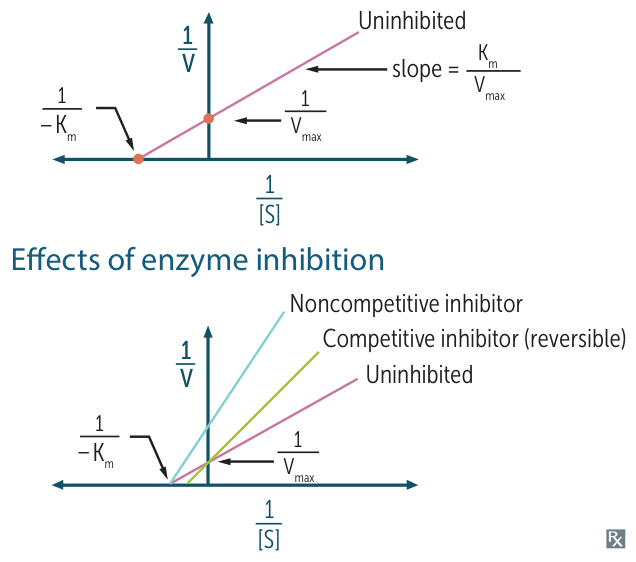
- Lineweaver-Burk Plot (Double-Reciprocal Plot)
- Linear plot of vs . Crucial for identifying inhibition types.
- X-intercept: represents . (Closer to 0 = larger /lower affinity).
- Y-intercept: represents . (Higher up = lower ).
- Slope: .
- USMLE “Cheat Sheet” for Lineweaver-Burk
- If lines cross at the Y-axis Competitive (Same , different ).
- If lines cross at the X-axis Non-competitive (Same , different ).
- If lines are Parallel Uncompetitive.
- If Y-intercept moves UP has decreased.
- If X-intercept moves RIGHT (closer to 0) has increased (lower affinity).
- Michaelis-Menten Plot
- Types of Inhibition (High-Yield)
- Competitive Inhibition
- Mechanism: Inhibitor binds to the Active Site, competing with substrate.
- Effect: Can be overcome by increasing .
- Kinetics:
- : Unchanged (sufficient outcompetes inhibitor).
- : Increases (lower affinity apparent).
- Lineweaver-Burk: Lines intersect at the Y-axis.
- Pharmacology Ex: Diazepam vs. GABA; Statins (HMG-CoA reductase).
- Non-competitive Inhibition
- Mechanism: Inhibitor binds to an Allosteric Site (not active site). Conformational change prevents catalysis.
- Effect: Cannot be overcome by increasing .
- Kinetics:
- : Decreases (functional enzyme pool is reduced).
- : Unchanged (affinity of remaining active enzymes is same).
- Lineweaver-Burk: Lines intersect at the X-axis.
- Pharmacology Ex: Ketoconazole (inhibition of CYP450).
- Uncompetitive Inhibition (Rarely tested directly, but know the pattern)
- Mechanism: Inhibitor binds only to the Enzyme-Substrate (ES) complex.
- Kinetics:
- : Decreases.
- : Decreases.
- Lineweaver-Burk: Lines are Parallel.
- Competitive Inhibition
- Cooperative Kinetics
- Sigmoidal Curve: Indicates cooperativity (e.g., Hemoglobin, PFK-1).
- Binding of one substrate molecule increases the affinity for the next.
- Hill Coefficient:
- : Positive cooperativity.
- : No cooperativity (Hyperbolic/Michaelis-Menten).
- : Negative cooperativity.