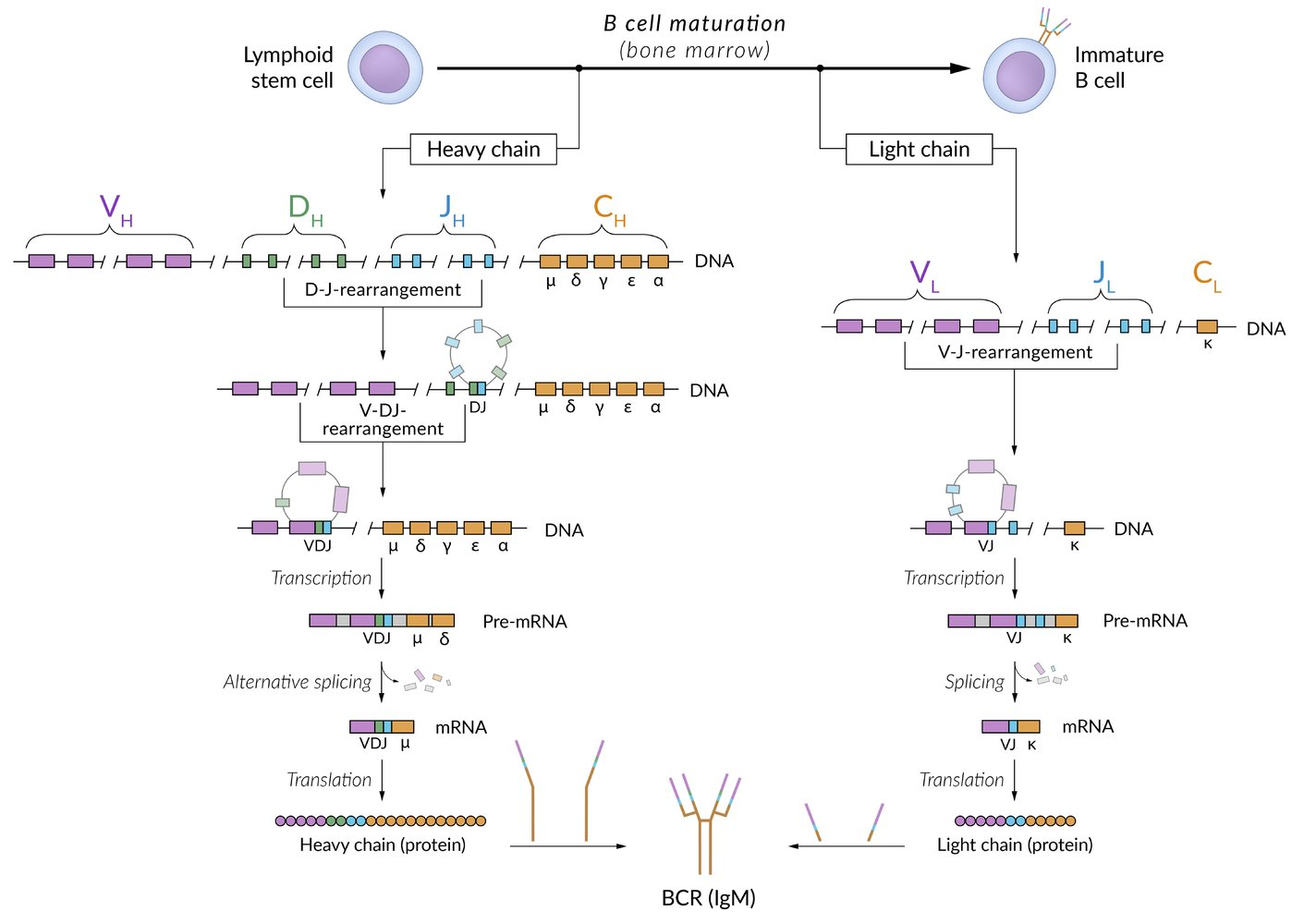
B-lymphocytes rearrange their V, D, and J gene segments to create diverse antibodies. T-lymphocytes, however, only rearrange their T-cell receptor genes. Consequently, in T-cells, the immunoglobulin genes remain in their original, unrearranged “germline” state.
 Immunotherapy medications often utilize fragments of a monoclonal immunoglobulin rather than the full immunoglobulin; because fragments are smaller, they typically have better tissue penetration and pharmacokinetics. Fab fragments contain a variable domain and the first constant region from a heavy and light chain; because they do not contain an Fc receptor, Fab fragments cannot trigger cell killing via complement or phagocytosis.
Immunotherapy medications often utilize fragments of a monoclonal immunoglobulin rather than the full immunoglobulin; because fragments are smaller, they typically have better tissue penetration and pharmacokinetics. Fab fragments contain a variable domain and the first constant region from a heavy and light chain; because they do not contain an Fc receptor, Fab fragments cannot trigger cell killing via complement or phagocytosis.
Hinge Region of Immunoglobulin
- Structure & Location
- A short, flexible stretch of amino acids located between the Cμ1 and Cμ2 domains of the heavy chains.
- Connects the two antigen-binding Fab arms to the Fc portion of the antibody.
- Present in IgG, IgA, and IgD.
- Absent in IgM and IgE, which instead have an additional constant domain (Cμ2) that confers flexibility.

- Composition
- Rich in proline residues, which prevent tight folding and provide flexibility.
- Rich in cysteine residues, which form interchain disulfide bonds that link the two heavy chains.
- Function
- Flexibility: Primary function. Allows the two Fab arms to move independently, enabling them to bind to epitopes separated by variable distances.
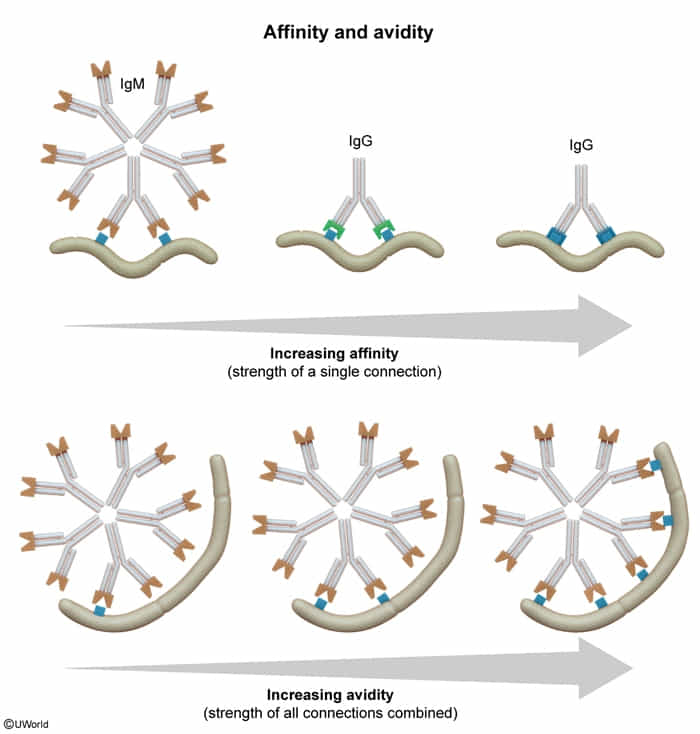
- Affinity = Attraction (Strength of a single bond)
- Avidity = Adhesion (Overall strength of all bonds combined)
- Complement Activation: Flexibility influences the ability of the Fc region to bind C1q and initiate the classical complement cascade.
- Fc Receptor Binding: Affects the interaction with Fc receptors on phagocytes, NK cells, and other immune cells, thus modulating effector functions like opsonization and ADCC.
- Site of Proteolytic Cleavage: Highly susceptible to cleavage by proteases (e.g., papain, pepsin), which is used experimentally to separate Fab and Fc fragments.
- Flexibility: Primary function. Allows the two Fab arms to move independently, enabling them to bind to epitopes separated by variable distances.
- Isotype & Subclass Variations (High-Yield) t
- IgG3: Has the longest and most flexible hinge region, making it the most potent IgG subclass for complement activation but also most susceptible to proteolysis.
- IgG2: Has a shorter, more rigid hinge due to multiple disulfide bonds, resulting in less flexibility.
- IgA1 vs. IgA2:
- IgA1: Found in serum; has a long, proline-rich hinge region. This makes it susceptible to cleavage by IgA proteases produced by bacteria like S. pneumoniae, H. influenzae, and Neisseria (S.H.i.N.), which is a key virulence mechanism.
- IgA2: Found in mucosal secretions; has a short hinge lacking the protease-sensitive site, making it resistant to cleavage.
- Clinical Correlations / Buzzwords
- Bacterial Immune Evasion: Cleavage of secretory IgA1 at the hinge region by bacterial IgA proteases allows pathogens to colonize mucosal surfaces.
- IgG Subclass Deficiency: Different hinge structures lead to different effector functions. IgG3 deficiency can lead to recurrent infections due to impaired complement activation.
Immunoglobulin types
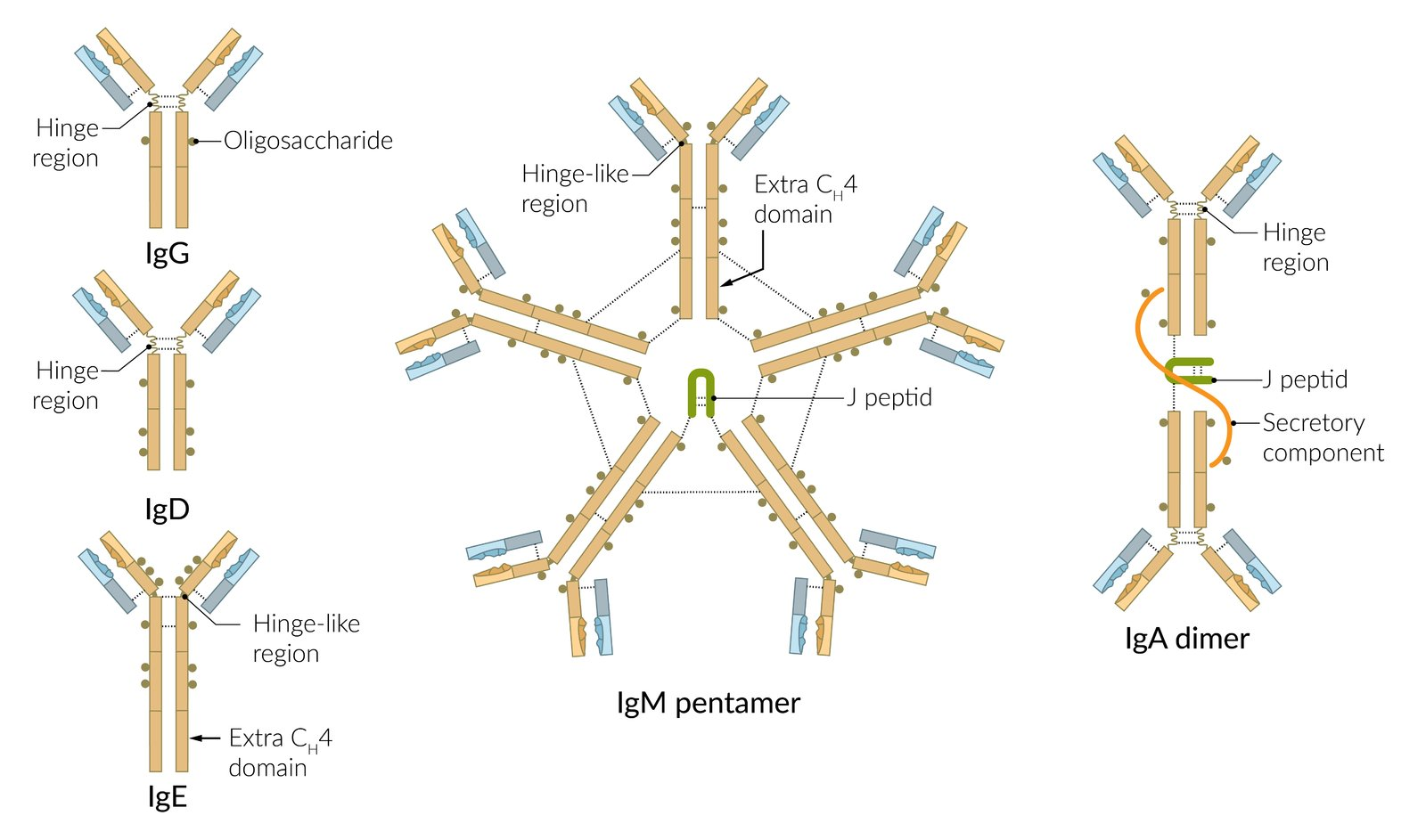
- IgM
- Blood group antibodies
- IgG
- The only immunoglobulin that can cross the placenta and thus conveys transient passive immunity to the child
- Neutralization of viruses and bacterial toxins, preventing the virus from entering host cells.
- IgA
- Found especially on mucosal surfaces and in bodily fluids like saliva, mucus, tears and breast milk
- Prevents binding of pathogens to mucous membranes of host cells
Mnemonic
IgG Greets the Growing fetus.
Antibody-dependent cell-mediated cytotoxicity (ADCC)
- Definition: an immune response in which Fc receptor-bearing immune effector cells (e.g., NK cells, eosinophils) bind to and lyse target cells that have specific antibodies attached to their surface antigens (pathogen or tumor-derived)
- Characteristics
- Allows innate immune cells to recognize pathogens that do not express pathogen-associated molecular patterns (PAMPs)
- Part of the immune response to parasites
- Effector cells
- NK cells (most common): interact with IgG-coated target cells and subsequently release cytotoxic substances (perforins, granzymes)
- Eosinophils: interact with IgE-coated helminths and subsequently release major basic proteins and other cytotoxic substances
- Other: monocytes, macrophages, neutrophils
- Mechanism
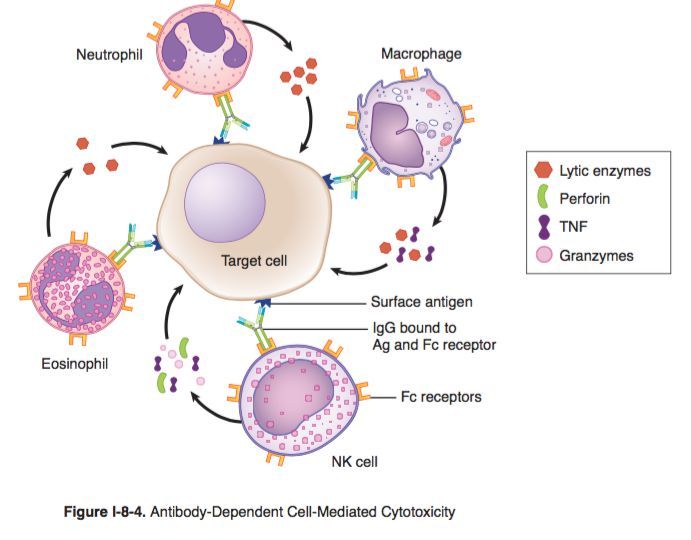
- Binding of antibodies (e.g., IgG or IgE) produced by B-cells to antigens on target cells (e.g., tumor cells, viruses, or parasites)
- Recognition and binding of the Fc portion of bound IgG or IgE by Fc receptors expressed on the surface of effector cells (e.g., CD16 Fc receptors on NK cells)
- Activation of signaling pathways in the effector cell (e.g., NK cell), leading to cytotoxic granule release
- Destruction of the target cell (e.g., via the perforin/granzyme cell death pathway)
- Clinical significance: monoclonal antibody therapy