Normal: Central pallor occupying ~1/3 of the cell diameter.
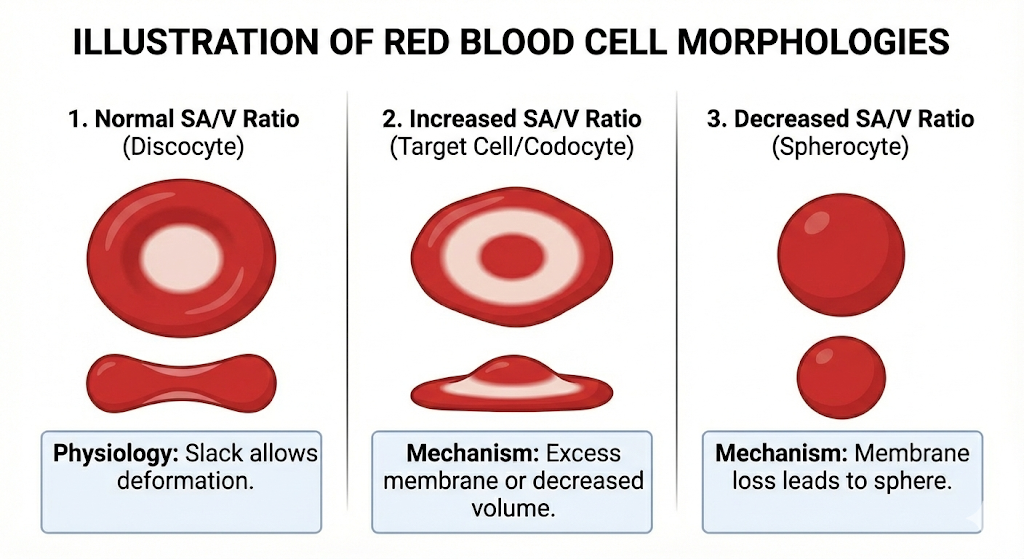
- ↑ SA/V Ratio Target Cell
- Mechanism: Excess membrane (“floppy bag”) or empty cytoplasm.
- Key Feature: “Bullseye” appearance.
- Lab: ↓ Osmotic Fragility (Resistant to bursting).
- Assoc: Thalassemia, Liver Disease, Asplenia.
- Normal SA/V Ratio Biconcave Disc
- Mechanism: Perfect balance allows folding through capillaries.
- Key Feature: Central pallor (~1/3 diameter).
- ↓ SA/V Ratio Spherocyte
- Mechanism: Membrane loss forces cell into tight sphere.
- Key Feature: Dense, NO central pallor.
- Lab: ↑ Osmotic Fragility (Bursts easily).
- Assoc: Hereditary Spherocytosis, Autoimmune Hemolysis.

Tip
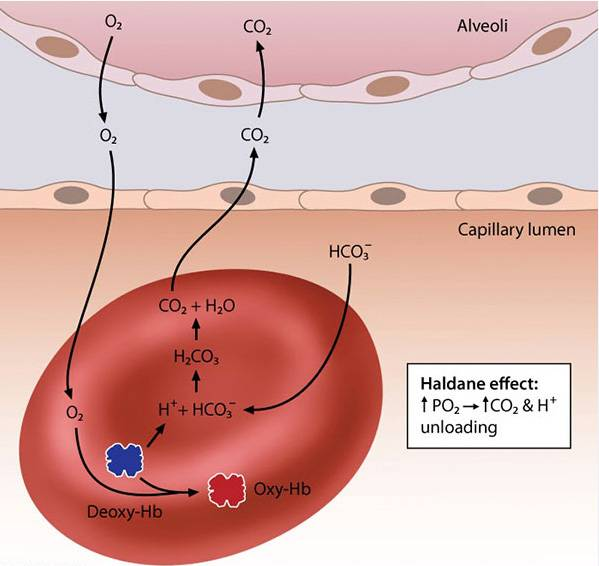
- The binding of O2 molecules to hemoglobin in the lungs has two consequences, known as the Haldane effect:
- The affinity of hemoglobin for CO2 is decreased, resulting in unloading of CO2 from hemoglobin (this accounts for a small percentage of overall CO2 in the blood and is not pictured above).
- The acidity of the hemoglobin molecule is increased; in response, protons (H+ ions) are released from the hemoglobin binding sites.
- The H+ ions combine with bicarbonate ions (the primary form of CO2 in the blood) in the lungs to facilitate the production of water (H2O) and CO2. The CO2 is then transferred to the alveoli and expired while oxygen is taken up by hemoglobin.
- In the peripheral tissues, high levels of CO2 create an increase in ambient acidity that shifts the hemoglobin dissociation curve to the right and facilitates the unloading of O2 (Bohr effect). The CO2 (and water) are converted into H+ and HCO3−. The H+ ions are carried by hemoglobin while the HCO3− is transferred to the plasma for transport back to the lungs.
Oxygen & Carbon Dioxide Transport
- Patho/Etiology
- Oxygen (O2): Primarily transported bound to hemoglobin (Hb) in red blood cells (RBCs) (~98%), with a small amount dissolved in plasma (~2%). The amount of dissolved O2 determines the partial pressure of O2 (PaO2).
- Carbon Dioxide (CO2): Transported in three forms:
- Bicarbonate (HCO3-): ~70-90%. CO2 enters RBCs and is converted by carbonic anhydrase to H2CO3, which dissociates into H+ and HCO3-. The HCO3- is then transported into the plasma in exchange for chloride ions (Cl-), a process known as the chloride shift.
- Carbaminohemoglobin: ~5-25%. CO2 binds directly to the N-terminus of globin chains on deoxyhemoglobin.
- Dissolved CO2: ~5-10%. CO2 is more soluble in blood than O2.
- Key Physiologic Effects
- Bohr Effect: In peripheral tissues, high CO2 and H+ levels promote the unloading of O2 from hemoglobin (causes a right shift in the O2-Hb curve). This is because H+ and CO2 stabilize the deoxygenated (T-state) form of hemoglobin, reducing its affinity for O2.
- Haldane Effect: In the lungs, the binding of O2 to hemoglobin promotes the release of CO2 and H+ from hemoglobin. This occurs because oxygenated hemoglobin is a stronger acid and has a lower affinity for CO2, facilitating CO2 release into the alveoli for exhalation.
- Clinical Correlations
- Anemia: Decreased Hb concentration leads to decreased total O2 content in the blood, even if PaO2 and SaO2 are normal.
- Carbon Monoxide (CO) Poisoning: CO binds to Hb with ~250x greater affinity than O2, forming carboxyhemoglobin. This reduces the number of available O2 binding sites and causes a left shift of the O2-Hb dissociation curve, impairing O2 unloading to tissues. Patients present with a normal PaO2 but low SaO2.
- Methemoglobinemia: Iron in the heme group is oxidized from Fe2+ (ferrous) to Fe3+ (ferric), which cannot bind O2. This also causes a left shift of the curve for the remaining functional hemoglobin.
- Cyanide Poisoning: Cyanide inhibits aerobic metabolism at the cellular level (cytochrome c oxidase), leading to an inability of tissues to utilize O2. Venous blood will have an unusually high O2 saturation.
Bohr effect
The O2 affinity of Hb is inversely proportional to the CO2 content and H+ concentration of blood. High CO2 and H+ concentrations (from tissue metabolism) cause decreased affinity for O2 → O2 that is bound to Hb is released to tissue (the O2-Hb dissociation curve is shifted to the right). HbO2 + H+ ⇄ H+Hb + O2 HbO2 + CO2 ⇄ Hb-COO- + H+ + O2
Haldane effect
The CO2 affinity of Hb is inversely proportional to the oxygenation of Hb. When Hb is deoxygenated (typically in peripheral tissue), uptake of CO2 is facilitated. When Hb is oxygenated (in high pO2, for example, in the lungs): Oxygenated Hb has a decreased affinity for CO2 → CO2 that is bound to Hb is released in the pulmonary arteries to diffuse into the alveoli (the O2-Hb dissociation curve is shifted to the left). Hb releases bound H+ → ↑ H+ shifts equilibrium to CO2 production (see equation above) → CO2 is exhaled in lungs
Mnemonic
Bohr for body, HaLdane for lung