De novo pyrimidine and purine synthesis
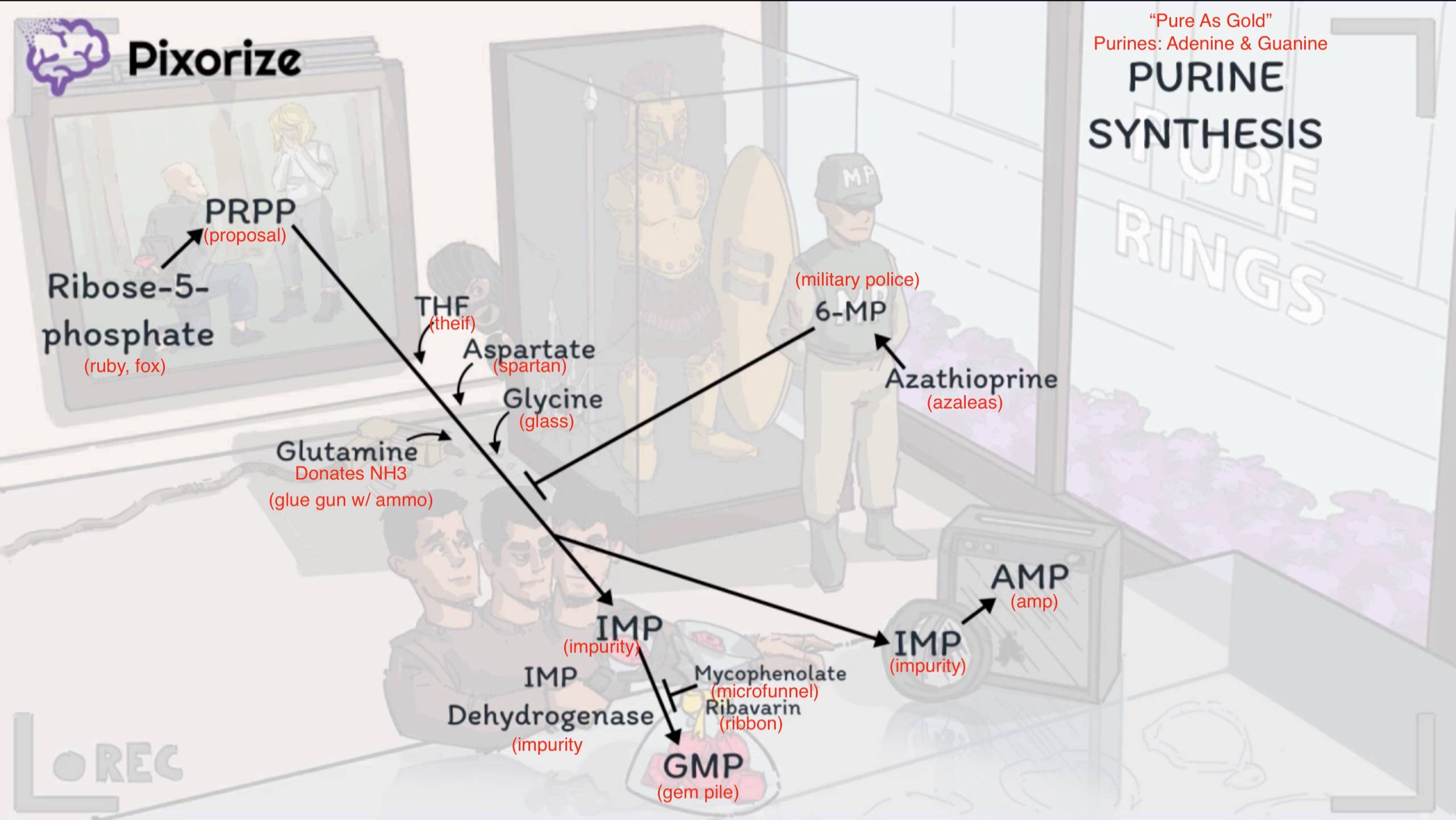
Purine synthesis

Pyrimidine synthesis


Folate acid pathway
Folate Pathway: The Basics
Folate (Vitamin B9) is converted to Tetrahydrofolate (THF), an essential coenzyme for:
- Purine synthesis (Adenine, Guanine)
- Thymidine synthesis (via N5,N10-methylene THF and thymidylate synthase)
- Amino acid metabolism
Key Enzymes & Inhibitors:
- Dihydropteroate Synthase (Bacteria only)
- Drug: Sulfonamides (e.g., Sulfamethoxazole - SMX)
- MoA: PABA analogs; block bacterial folic acid synthesis.
- Use: UTIs, PJP (often with Trimethoprim).
- AEs: Hypersensitivity (SJS), photosensitivity, crystalluria, G6PD hemolysis, kernicterus.
- Drug: Sulfonamides (e.g., Sulfamethoxazole - SMX)
- Dihydrofolate Reductase (DHFR)
- Drug: Trimethoprim (TMP) (Primarily bacterial DHFR)
- MoA: Inhibits bacterial DHFR ⟹
THF. - Use: UTIs, PJP (synergistic with SMX = TMP-SMX/Co-trimoxazole).
- AEs: Megaloblastic anemia, leukopenia, hyperkalemia. (Leucovorin can reduce bone marrow effects).
- MoA: Inhibits bacterial DHFR ⟹
- Drug: Methotrexate (MTX) (Human DHFR)
- MoA: Inhibits human DHFR ⟹
THF ⟹ DNA/RNA synthesis. - Use: Cancer, rheumatoid arthritis, psoriasis, ectopic pregnancy, immunosuppression.
- AEs: Myelosuppression (dose-limiting), mucositis, hepatotoxicity, pulmonary fibrosis, nephrotoxicity, teratogenic.
- Leucovorin (Folinic Acid) Rescue: Bypasses DHFR block in normal cells to reduce MTX toxicity.
- Leucovorin is 5-formyl-tetrahydrofolate. This means its core pteridine ring structure is already in the fully reduced (tetrahydro) state, the same state that DHFR aims to achieve.
- MoA: Inhibits human DHFR ⟹
- Drug: Pyrimethamine (Protozoal DHFR)
- MoA: Inhibits protozoal DHFR.
- Use: Toxoplasmosis (with sulfadiazine), malaria.
- AEs: Megaloblastic anemia. (Leucovorin can reduce bone marrow effects).
- Drug: Trimethoprim (TMP) (Primarily bacterial DHFR)
- Thymidylate Synthase (Requires N5,N10-methylene THF from folate pathway)
- Drug: 5-Fluorouracil (5-FU)
- MoA: Pyrimidine analog; active metabolite FdUMP forms a complex with thymidylate synthase and N5,N10-methylene THF, inhibiting dTMP ("thymineless death").
- Use: Solid tumors (e.g., colorectal, breast, pancreatic).
- AEs: Myelosuppression, mucositis/diarrhea, hand-foot syndrome, photosensitivity.
- Leucovorin (Folinic Acid) Potentiation: Enhances 5-FU efficacy by stabilizing the inhibitory complex (different role than with MTX).
- Note: DPD deficiency increases 5-FU toxicity.
- Drug: 5-Fluorouracil (5-FU)
| Drug | Target Enzyme/Process | Primary Use(s) | Key Adverse Effects | Leucovorin Interaction |
|---|---|---|---|---|
| Sulfonamides | Bacterial Dihydropteroate Synthase | UTIs, PJP (bacterial infections) | Hypersensitivity, photosensitivity, crystalluria, G6PD hemolysis | N/A |
| Trimethoprim | Bacterial Dihydrofolate Reductase (DHFR) | UTIs, PJP (bacterial infections, with SMX) | Megaloblastic anemia, leukopenia, hyperkalemia | Rescue (bone marrow) |
| Methotrexate | Human Dihydrofolate Reductase (DHFR) | Cancer, autoimmune disease, ectopic pregnancy | Myelosuppression, mucositis, hepatotoxicity, teratogenic | Rescue (reduces toxicity) |
| Pyrimethamine | Protozoal Dihydrofolate Reductase (DHFR) | Toxoplasmosis, malaria | Megaloblastic anemia | Rescue (bone marrow) |
| 5-Fluorouracil | Thymidylate Synthase (via FdUMP & N5,N10-methylene THF complex) | Solid tumors (e.g., colorectal cancer) | Myelosuppression, mucositis, diarrhea, hand-foot syndrome | Potentiation (increases efficacy) |
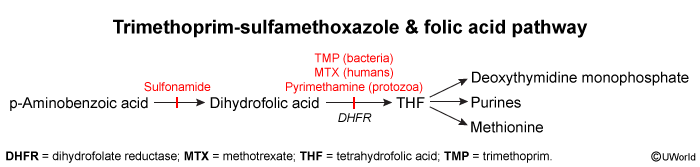

Para-aminobenzoic acid (PABA) is a folic acid precursor in prokaryotes. Sulfonamide antibiotics are chemical analogues of PABA that inhibit the enzyme dihydropteroate synthetase, preventing bacterial conversion of PABA to folic acid. Humans lack the ability to convert PABA to folic acid and require dietary folate.

The enzyme thymidylate synthase is responsible for converting deoxyuridine monophosphate (dUMP) to deoxythymidine monophosphate (dTMP). Although most enzymes involved in one-carbon metabolism maintain folate in its active tetrahydrofolate form, thymidylate synthase is unique in that it oxidizes 5,10-methylenetetrahydrofolate to dihydrofolate. This makes de novo thymidine synthesis particularly susceptible to folate-deficient conditions because tetrahydrofolate must be continuously regenerated by dihydrofolate reductase.